Recall of five valsartan-containing pharmaceutical products (with photos)
*************************************************************************
The affected products are:
| Product | Hong Kong Registration Number | Registration certificate holder |
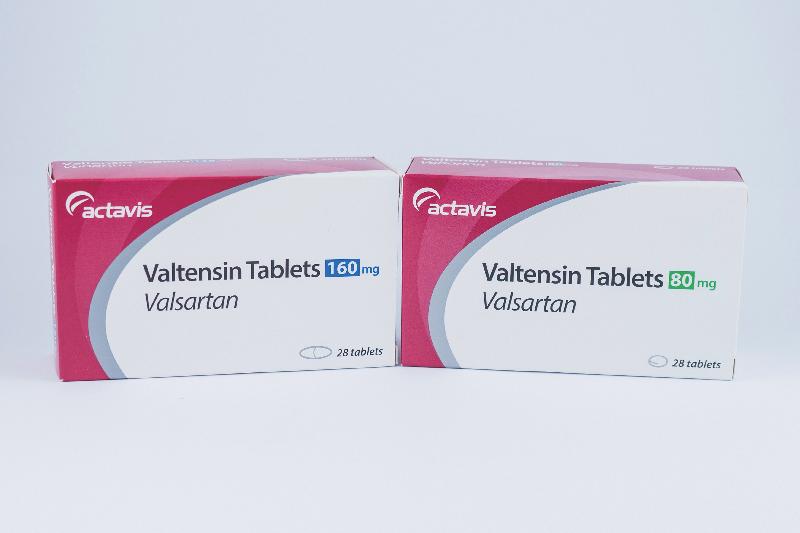
| Valtensin 160mg tablets | HK-61786 | Actavis |
| Valtensin 80mg tablets | HK-61787 | Actavis |
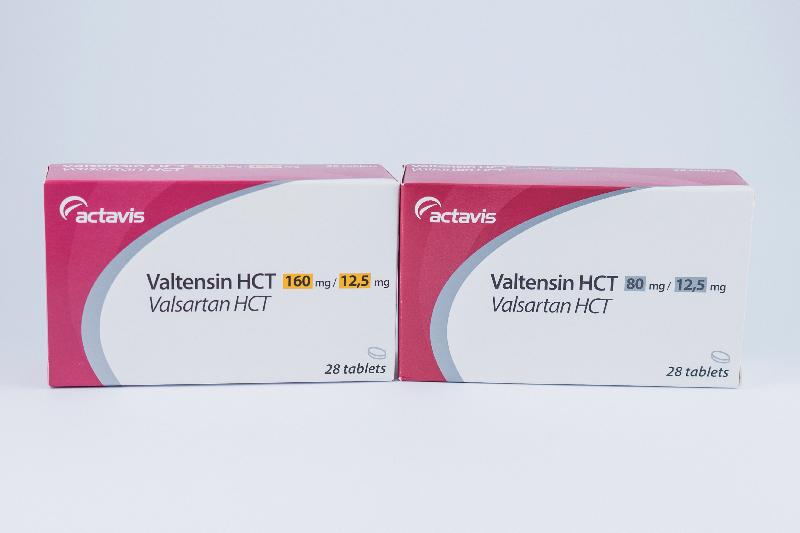
| Valtensin HCT tablets 160/12.5mg | HK-61784 | Actavis |
| Valtensin HCT tablets 80/12.5mg | HK-61785 | Actavis |
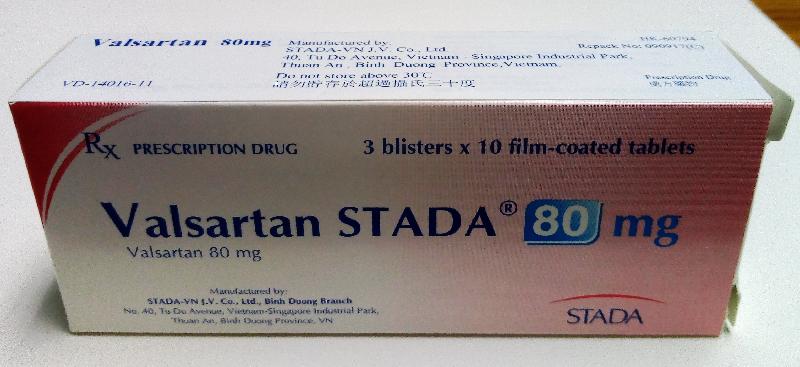
| Valsartan Stada 80mg tablets | HK-60794 | HK Medical |
The DH, through its surveillance system, noted that the raw material valsartan produced by a manufacturer in the Mainland and used in certain pharmaceutical products as an active ingredient was found to contain an impurity, N-nitrosodimethylamine (NDMA). NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests.
So far, the above products registered in Hong Kong are confirmed by the wholesalers to contain the affected raw material. The DH's investigation is continuing.
According to the preliminary investigation of the Mainland manufacturer, the presence of NDMA in the raw material valsartan is believed to be related to its production method.
Valsartan-containing products are prescription medicines used to treat hypertension and heart failure. They are available as single ingredient products or in combination with other active ingredients.
According to Actavis and HK Medical, the above products have been supplied to local private doctors and pharmacies. The products Valtensin 80mg and 160mg tablets have also been supplied to the Hospital Authority.
Both companies have set up hotlines (Actavis: 3188 4288; HK Medical: 2806 3112) to answer related enquiries.
So far, the DH has not received any adverse reactions related to the above affected products.
Patients who are taking the above products should not stop taking the medicines, but should seek advice from their healthcare professionals for appropriate arrangement.
Ends/Friday, July 6, 2018
Issued at HKT 20:26
Issued at HKT 20:26
NNNN