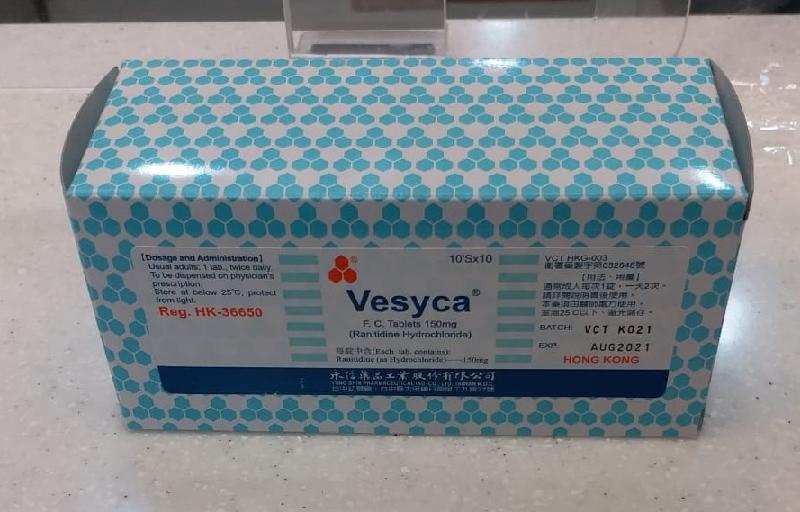
Recall of Vesyca FC Tablets 150mg (with photo)
**********************************************
The DH received notification from the wholesaler that the manufacturer of the product in Taiwan is recalling the above product because it might contain an impurity, namely N-nitrosodimethylamine (NDMA). As a precautionary measure, the wholesaler is voluntarily recalling the above product from the local market.
NDMA is classified as a probable human carcinogen based on results from laboratory tests. The DH noted that certain ranitidine-containing products were found to contain NDMA in other countries, therefore a letter was sent to healthcare professionals on September 18, 2019, notifying them about the issue. The DH will closely monitor the development of the issue and any safety updates of the drug issued by overseas drug regulatory authorities for consideration of action deemed necessary.
The above product, containing ranitidine, is an over-the-counter medicine used for the treatment of gastric diseases. According to the wholesaler, the product has been supplied to private doctors and pharmacies.
The wholesaler has set up a hotline (2637 5131) to answer related enquiries.
"So far, the DH has not received any adverse reaction reports in connection with the product. The DH will closely monitor the recall," a spokesman for the DH said.
"Patients who are taking the above product should seek advice from their healthcare professionals for appropriate management. There are alternative medicines available on the market with similar indications," the spokesman added.
Ends/Thursday, August 27, 2020
Issued at HKT 15:40
Issued at HKT 15:40
NNNN