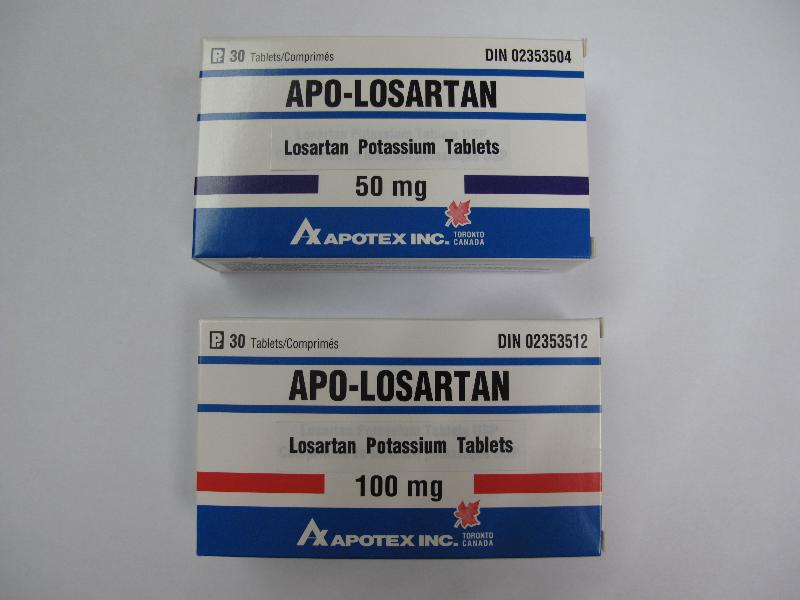
Batch recall of four losartan-containing pharmaceutical products (with photos)
******************************************************************************
The affected products are:
| Product | Hong Kong Registration Number | Batch Number |
| Apo-Losartan Tablets 50mg | HK-61932 | NK 1253 |
| Apo-Losartan Tablets 100mg | HK-61933 | NG 2092 NH 5932 NL 1460 |
| Apo-Losartan/HCTZ Tablets 50mg/12.5mg | HK-62635 | NZ 8848 NL 1441 |
| Apo-Losartan/HCTZ Tablets 100mg/25mg | HK-62634 | NZ 8845 |
Through its surveillance system, the DH noted that Health Canada (the Canadian regulatory authority) is advising that multiple lots of losartan-containing products are being voluntarily recalled because of the potential for an impurity, N-Nitroso-N-methyl-4-aminobutyric acid (NMBA). NMBA is a potential human carcinogen.
Losartan-containing products are prescription medicines used to treat hypertension. According to Hind Wing, the affected batches of the above products have been supplied to private doctors and pharmacies.
"So far, the DH has not received any adverse reactions related to the above affected products," a DH spokesman said.
Hind Wing has set up a hotline (2541 5731) to answer related enquiries. The DH will closely monitor the recall.
The spokesman advises that patients who are taking the above products should not stop taking the medicines, but should seek advice from their healthcare professionals for appropriate arrangement.
Ends/Monday, March 11, 2019
Issued at HKT 20:10
Issued at HKT 20:10
NNNN