Replacement arrangements for anti-hypertensive drug Valtensin (with photos)
***************************************************************************
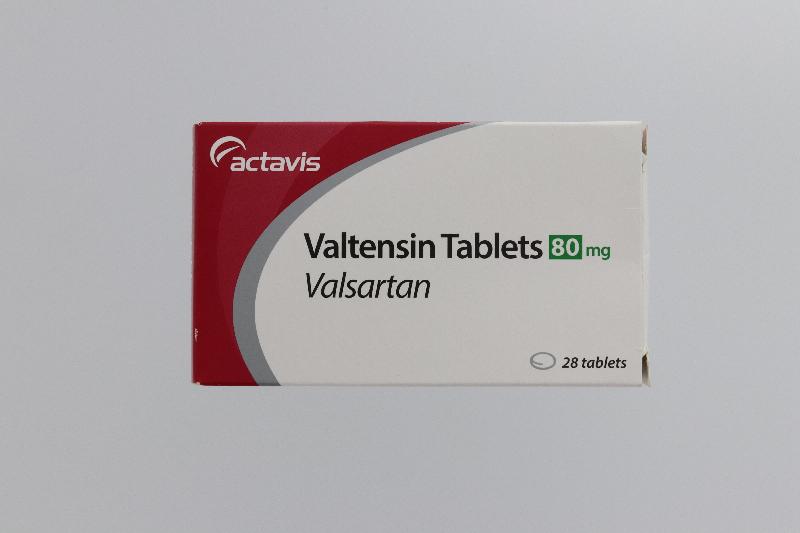
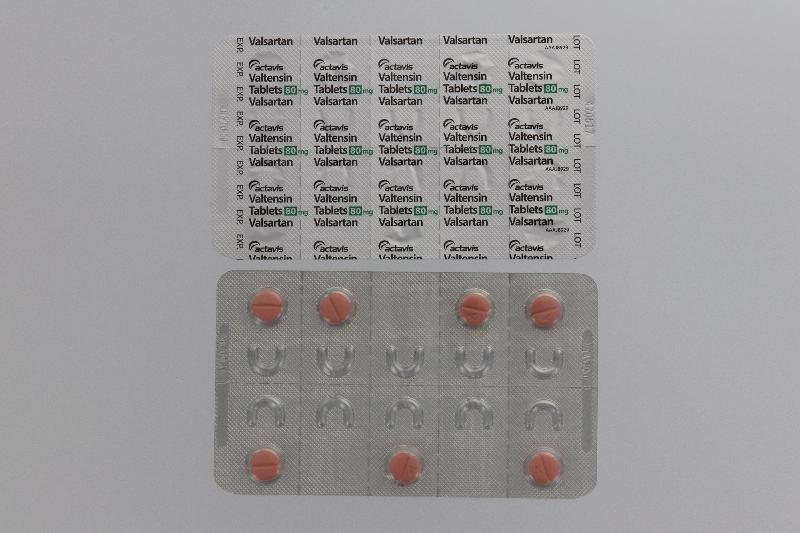
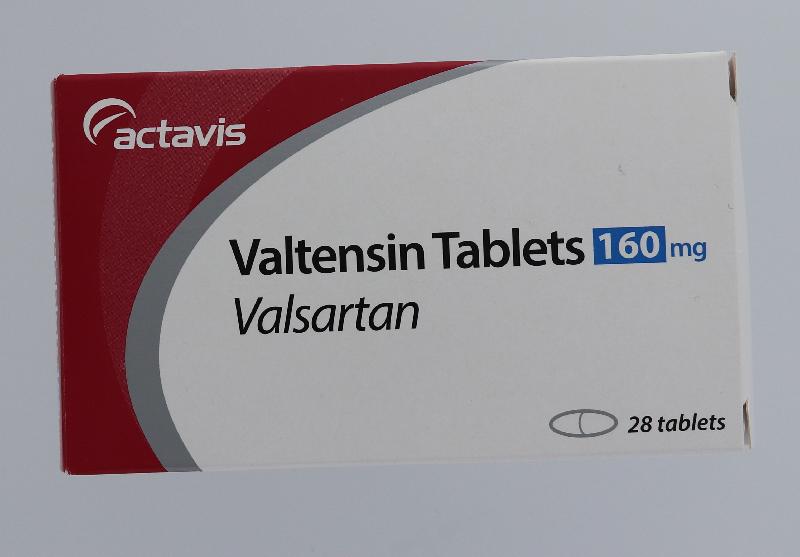
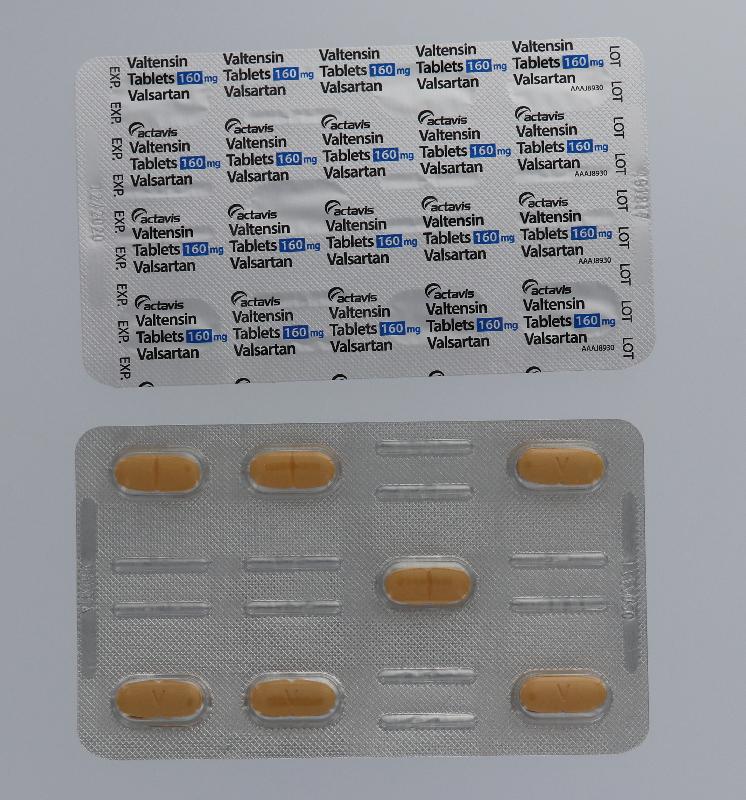
The Hospital Authority (HA) spokesperson today (August 1) announced that in response to the earlier announcement by the Department of Health (DH) on the recall of the anti-hypertensive drug Valtensin (80mg tablets and 160mg tablets, containing the active drug ingredient valsartan) as it was found to contain an impurity, N-nitrosodimethylamine, an optional drug replacement will be offered to public hospital patients.
Starting from this Saturday (August 4), the HA will arrange drug replacement counters at 18 designated public hospitals for two weeks for public hospital patients who had been dispensed with the drug Valtensin on or before July 6 to opt for replacement with alternative supply of valsartan tablets from other suppliers.
The spokesperson pointed out that all public hospitals have already ceased to dispense the drug concerned, Valtensin (80 mg tablets and 160mg tablets), from July 6 in response to the DH recall. According to the assessment of clinical experts, there is no immediate risk to patients in continued intake of the drug. The experts are also of the view that an abrupt stop in taking the drug might cause an adverse effect on the patient's condition. Hence, patients who were dispensed with the drug are recommended to not stop taking it until they attend their next follow-up consultations, in which the patients would be prescribed the appropriate replacement medication after medical assessment.
"The HA has recently secured adequate quantities of alternative supplies from overseas, which is sufficient to replace the quantities previously dispensed to patients. Therefore, patients can now opt for drug replacement before their next follow-up appointment," the spokesperson said.
"Patients who choose to replace their drugs on hand should bring along their ID card with the drugs (see photos), or ask their delegate to bring along a copy of the patient's ID card and the drugs on hand, and go to any of the 18 designated counters as listed below during the period from August 4 to 17 for drug replacement after verification by the pharmacist on their respective dispensing records. No payment is required for the drug replacement."
The spokesperson stressed that since the drug replacement service will last for two weeks from 9am to 9pm on a daily basis, patients can take their time to visit for drug replacement. Furthermore, patients who have their follow-up appointments at clinics in outlying islands will individually be notified on the drug replacement arrangements. The HA will also continue to closely liaise with the DH on the matter.
The 18 designated drug replacement counters are listed below:
| Hospital | Location of Drug Replacement Counter | Enquiry Number |
| Pamela Youde Nethersole Eastern Hospital | Main Pharmacy, Pamela Youde Nethersole Eastern Hospital | 6460 0553 |
| Ruttonjee Hospital | Pharmacy, G/F, Admin Building, Ruttonjee Hospital | 2291 2079 |
| Queen Mary Hospital | Central Pharmacy, 1/F, Block S, Queen Mary Hospital | 2255 6649 |
| Tung Wah Hospital | Pharmacy, 1/F, Centenary Building, Tung Wah Hospital | 2589 8111 |
| Queen Elizabeth Hospital | Pharmacy, 2/F, Ambulatory Care Centre, Queen Elizabeth Hospital | 5278 2982 |
| Kwong Wah Hospital | Pharmacy, 1/F, North Wing, Kwong Wah Hospital | 3517 2700 |
| Our Lady of Maryknoll Hospital | Pharmacy, LG, Outpatient Block, Our Lady of Maryknoll Hospital | 2354 0500 |
| United Christian Hospital | Pharmacy, G/F, Block S, United Christian Hospital | 3949 4000 |
| Tseung Kwan O Hospital | Pharmacy, LG/F, Hospital Main Block, Tseung Kwan O Hospital | 2208 0111 |
| Princess Margaret Hospital | Pharmacy, LG2, Block A, Princess Margaret Hospital | 2370 0980 |
| Caritas Medical Centre | Pharmacy, LG1, Wai Ming Block, Caritas Medical Centre | 3408 6348 |
| North Lantau Hospital | Pharmacy, 3/F, North Lantau Hospital | 3467 7340 |
| Yan Chai Hospital | Pharmacy, 1/F, Block C, Yan Chai Hospital | 2417 8383 |
| Prince of Wales Hospital | Main Pharmacy, 1/F, Main Clinical Block and Trauma Centre, Prince of Wales Hospital | 5569 9855 |
| Alice Ho Miu Ling Nethersole Hospital | Pharmacy, B1, Block C, Alice Ho Miu Ling Nethersole Hospital | 2689 2699 |
| North District Hospital | Out-patient Pharmacy, North District Hospital | 2683 7561 |
| Tuen Mun Hospital | Pharmacy, G/F, Ambulatory Care Centre, Tuen Mun Hospital (Saturday and Sunday) Pharmacy, G/F, Main Block, Tuen Mun Hospital (Monday to Friday) |
2468 6872 |
| Pok Oi Hospital | Pharmacy, M/F, Central Wing, Pok Oi Hospital | 2401 4592 |
Drug replacement period: August 4 (Saturday) to August 17 (Friday)
Service hours: 9am to 9pm daily
Ends/Wednesday, August 1, 2018
Issued at HKT 19:28
Issued at HKT 19:28
NNNN