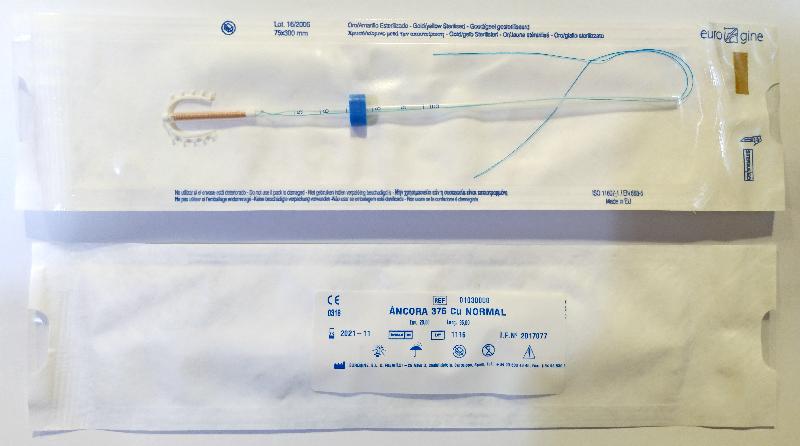
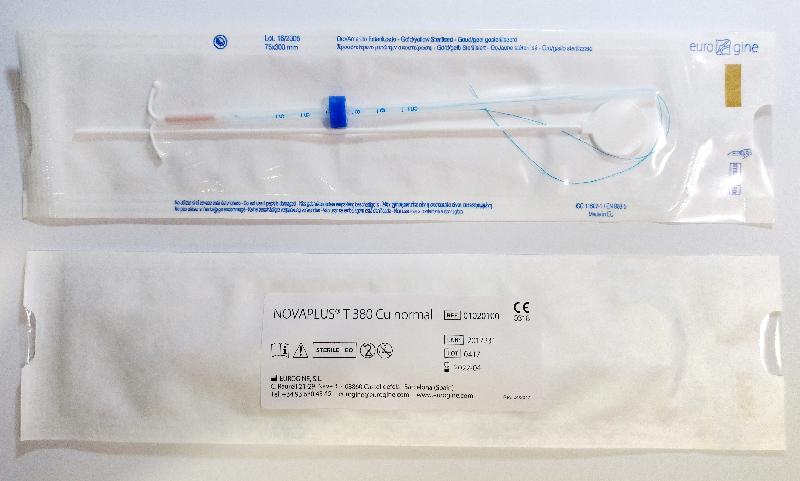
Recall of Eurogine SL intrauterine devices (with photos)
********************************************************
The DH received notification from the local supplier of the recall involving two affected models and five affected lots, namely Ancora 375 Cu Normal (Lots: 0614 and 1116) and Novaplus T380 Cu Normal (Lots: 0216, 0217 and 0417).
According to the manufacturer, the issue was caused by defective manufacturing of the raw material. The efficacy of the IUD is not affected and premature removal of the device is not recommended. When getting the IUD removed, it is recommended that the healthcare professional performs a slow and constant traction when pulling the threads.
According to the local supplier, around 1 400 units of the affected IUDs were distributed to various organisations in Hong Kong, including private hospital and clinics, as well as the Maternal and Child Health Centres (MCHCs) under the DH's Family Health Service. The supplier has notified all affected organisations and replacement of the affected products is underway.
"The DH has informed relevant organisations about the recall and will continue to liaise with the local supplier on necessary follow-up actions.
"So far, the DH has not received any reports of adverse incidents related to the affected devices in Hong Kong. Women should consult the doctor who performed the IUD insertion, if in doubt," a spokesman for the DH said.
Regarding the affected IUDs with the DH, among the 500 affected units distributed to MCHCs, 62 have been used on clients attending family planning service. The DH will bring the matter to these clients' attention. Counselling and consultation will be offered if necessary.
Ends/Wednesday, March 14, 2018
Issued at HKT 18:36
Issued at HKT 18:36
NNNN