
King-Man HO
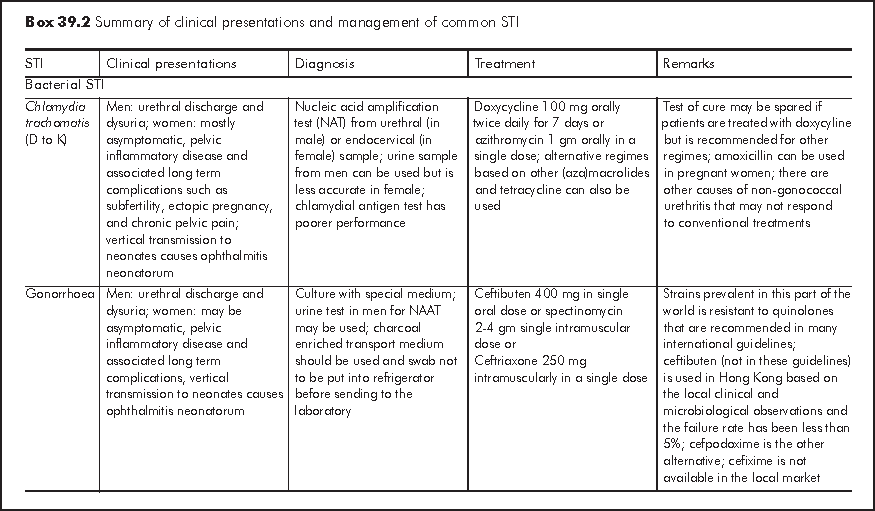
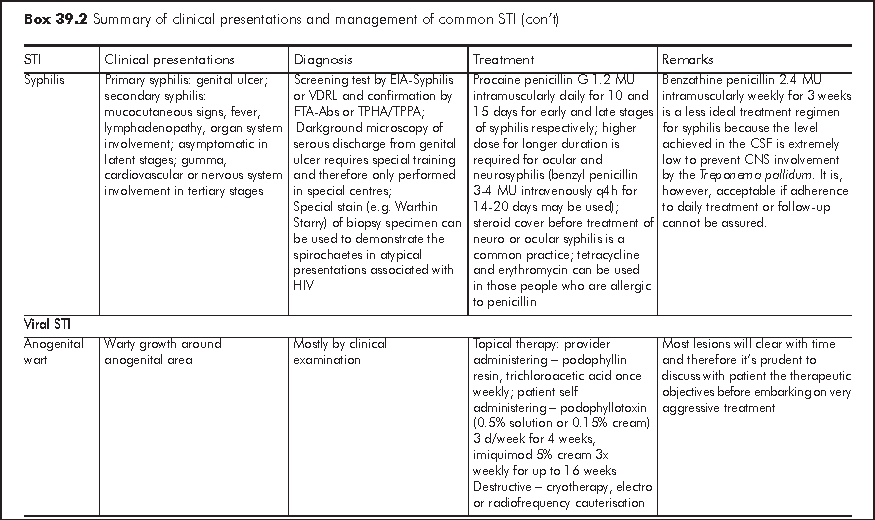
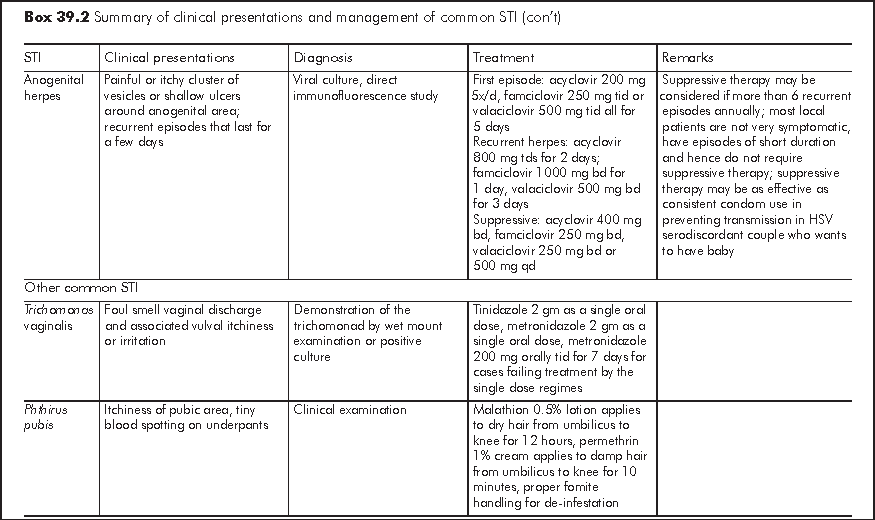
Sexually transmitted infections (STI) can broadly be defined as infections that are transmissible through sexual contact. Many enteric infections may also be grouped under this category because of their faecal oral route of transmission sometimes associated with sexual activities. For simplicity of discussion, STI in this chapter refers to infections that are predominantly transmitted through sexual contact. Common viral STI include anogenital herpes, anogenital human papilloma virus (HPV) infection. Common bacterial STI include syphilis, gonorrhoea, and Chlamydia trachomatis (serovar L1, 2, 3 that cause lymphogranuloma venereum and D to K that cause urethritis and cervicitis). Other common STI agents include Trichomonas vaginalis, and Phthirus pubis.
The World Health Organization (WHO) estimated that there were more than 300 million new cases of the 4 treatable STI (syphilis, gonorrhoea, Chlamydia trachomatis and Trichomonas vaginalis) in the world each year. In China, the incidence rates of the eight notifiable STI had increased from 13.85 per 100,000 in 1990 to 58.15 per 100,000 in 2002.1 Cities along the coastline were the most heavily affected. In Hong Kong, the Government Social Hygiene Service (SHS), the major public service provider for people with STI, recorded about 18,000 new cases of STI and genital tract infections in 2005. The top five were non-gonococcal infection and non-specific genital tract infection, anogenital warts, gonorrhoea, syphilis and anogenital herpes in descending order. The seroprevalence of syphilis among pregnant women attending local antenatal services was about 0.47% in 20042 and the seroprevalence of Herpes simplex 2 virus was estimated to be about 10%.
There are two major approaches in the case management of STI - aetiological and syndromic.3 The general principles of STI management are in Box 39.1.

Aetiological approach is the conventional way of managing STI according to the classic microbiological principles i.e. treatment according to the microbe identified in the laboratory. Conventionally, this approach involves attendance at clinic, collection of samples, and their delivery for testing at designated laboratories. In the recent years, there have been quite a number of new STI diagnostics emerging in the market. These include point-of-care tests and tests on self collected samples. Correct application and proper interpretation of these tests are of utmost importance. There is wide variation of test performance in terms of sensitivity and specificity, and the results could also be operator dependent. It is important that clinicians requesting these tests familiarise themselves with their performance, and should offer appropriate advice to clients accordingly.
Syndromic management is an alternative approach to STI as the aetiological approach may not be always applicable. In resource poor settings, for example, the cost, technological know-how and quality control of concerned STI tests may limit the access and accuracy of these tests. In hard-to-reach populations, aetiological approach may not be feasible, even in apparently developed countries. In syndromic case management, people with genital symptomatology and clinical feature are grouped under a few syndromes for which the most likely microbes are considered. Patients are given relevant drug treatment and counselling according to a standard set of algorithms developed in the unique setting of the region. Widely adopted algorithms are those for management of urethral discharge, genital ulcer, vaginal discharge, and lower abdominal pain syndromes. The World Health Organization (WHO) has developed prototypes of these algorithms to be modified and adapted by individual place. Some of these algorithms are validated in field studies, which are practicable albeit with variable performance.4 Urethral discharge syndrome algorithm is regarded as the best performed while vaginal discharge algorithm the most variable. The syndromic case management approach has been adopted to reduce STI and reproductive tract infections in the first community based HIV prevention programme in Africa.5-8
In Hong Kong, an aetiological approach is adopted by the Social Hygiene Service for patients who attend the clinics. Management of the common STI is summarised in Box 39.2. Only those laboratory tests and treatment readily available in Hong Kong are included. The algorithm for managing STI, modelled on the approach of the Social Hygiene Clinic is at shown at the back of this chapter. On the other hand, syndromic algorithms have also been developed by the Centre for Health Protection for local application with the intention of supporting the primary care sector in managing people with STI related presentations.9
Both ulcerative and non-ulcerative STI facilitate HIV transmission by a factor of 2-5 times, more for ulcerative comparing to non-ulcerative ones. On the other hand, STI is not uncommonly found in people with HIV infection. In Hong Kong, a cross sectional study conducted in 2005 revealed that 5.1% of sexually active people attending an HIV clinic had either gonococcus or Chlamydia trachomatis infection from urine PCR screening.10 These observations highlight the importance of STI screening, diagnosis and treatment for HIV-infected people.
The clinical presentations, natural course and management of STI in people with HIV are usually the same as those non HIV-infected people. Nevertheless, atypical presentations and interactions of significant clinical or public health interest have been extensively reported in the literature.
Increased genital shedding of both HIV and herpes simplex virus type 2 (HSV-2) in women co-infected with both viruses was reported. Shedding of HSV-2 in co-infected women is twice as high in level as those not infected with HIV. Moreover, women with HIV are more likely to have higher HIV RNA copy number in their blood.11 There are ongoing studies of using suppressive acyclovir as a therapy to lower HIV load and transmission. Atypical presentations such as large atypical anogenital ulcer recalcitrant to conventional treatment and hyperplastic or nodular growth were reported in the literature.
In persons with HIV, the recommended regimes for daily suppressive therapy are: acyclovir 400-800 mg orally twice to three times a day or famciclovir 500 mg orally twice a day, or valaciclovir 500 mg orally twice a day. The recommended regimes for episodic treatment are: acyclovir 400 mg orally three times a day for 5-10 days or famciclovir 500 mg orally twice a day for 5-10 days, or valaciclovir 1 gm orally twice a day for 5-10 days. The dosages are higher than those in persons without HIV infection.
People infected with HIV are more likely to have a detectable human papillomavirus (HPV) infection regardless of the subtype.12 Moreover, they stand a higher chance of having persistent infection with high-risk HPV types (HR-HPV).13 Women with HIV are prone to have cellular atypia in their cervical screening samples, nevertheless the degree to which immunosuppression affects atypia is not yet well defined.14
Anogenital wart is not uncommon among men having sex with men (MSM). There were reports of increased risk of anal carcinoma among MSM with HIV infection.15 Although screening for anal carcinoma in HIV infected MSM has been advocated by some investigators, there is no standard recommendation on anal intraepithelial neoplasia screening and management. Interestingly, the risk for abnormal anal "Pap smear" is better associated with anal sex and homosexual orientation than level of immunosuppression defined by CDC class or CD4 count.16 Atypical presentations such as extensive growth recalcitrant to treatment, accelerated progression to high grade lesions and even cervical cancer or anal cancer after infection with HR-HPV were reported in the literature.17
In a cross sectional study conducted in an HIV clinic in Hong Kong in 2001, the seroprevalence rate of syphilis among people with HIV was 11%18 comparing to the general population average of less than 0.5%. There were reports of the resurgence of syphilis in certain ethnic subgroups in the US.19 The effectiveness of HAART may have underlay the rising trend of syphilis in some countries.
Diagnosis of syphilis often poses a challenge to the clinician, beginning with the choice from a myriad of tests available. The following are common ones:
Dark ground microscopy
Primary syphilis: Spirochaetes can be detected from serum of chancres which can be examined by dark ground microscopy. To improve the yield, examination performed for three consecutive days is usually practised in clinics equipped with the technological know-how. The test is less reliable for oral lesion because of the presence of other commensal spirochaetes.
Secondary syphilis: Serum from ulcers and condylomata lata can be examined by dark ground microscopy.
Skin biopsy
Serology
Serology testing is the mainstay of laboratory testing for syphilis. Exceptions are primary and secondary syphilis where the spirochaete Treponema pallidum can directly be identified (as aforementioned). Only the appropriate application of serological tests for syphilis can lead to the correct diagnosis of syphilis. The concepts of using Venereal Diseases Research Laboratory carbon antigen test (VDRL) alone for screening and Fluorescent treponemal antibody absorption test (FTA-ABS) as a "good standard" for confirmation should be revised. With the introduction of syphilis enzyme immunoassay (EIA) test, practitioners should familiarise themselves with this newer treponemal test.
Serological tests for syphilis are classified into two categories:
non-treponemal tests e.g. VDRL and rapid plasma reagin (RPR), and treponemal
tests e.g. FTA-ABS, Treponema pallidum haemagglutination assay (TPHA)
test, Treponema pallidum particle agglutination test (TPPA), and
syphilis enzyme immunoassay (EIA).
The principle of the application of syphilis serology is the detection
of treponemal antibody by a screening test, followed by confirmation with
an additional test. The confirmation test should be different from the screening
test in their mechanisms, with equivalent sensitivity and ideally better
specificity. The new approach of the Public Health Laboratory Centre, Department
of Health20 and conventional testing algorithm21
are shown in Box 39.3.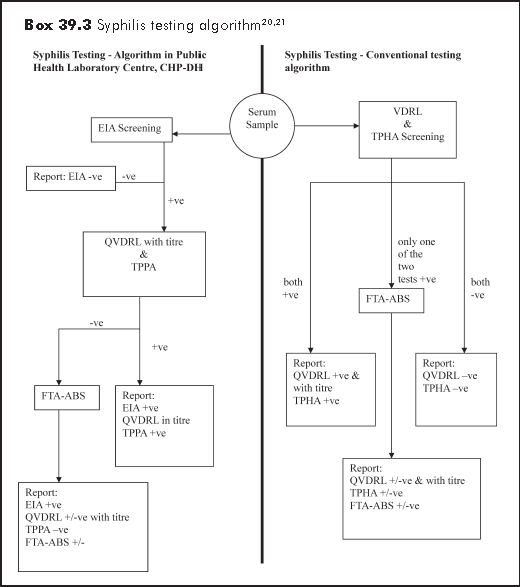
Depending on the serological test used, syphilis can be confirmed by serology as early as 2 weeks after infection. The local Social Hygiene Clinic recommends second serology at 3 months as by then almost all infected cases will have positive serology.
(i) Non-treponemal (reaginic) test
Venereal Diseases Research Laboratory carbon antigen test (VDRL) is inexpensive, and readily quantifiable that allows monitoring of disease activity. However, the test is limited by biological false positives, and poor sensitivity in late syphilis. Prozone phenomenon, false negative result as a result of very high titre antibodies, is excluded by specimen dilution. This is the only test that allows serological diagnosis of re-infection in people with treated syphilis.
(ii) Treponemal test
Fluorescent treponemal antibody absorption test (FTA-ABS) is a treponemal test that has been adopted as a confirmatory test in syphilis. It becomes positive earlier than VDRL. As test interpretation is operator dependent, quality control of the reporting laboratory is of importance in maintaining the performance of this test.
Treponema pallidum particle agglutination test (TPPA) has already replaced Treponema pallidum haemagglutination assay (TPHA) in many laboratories. Evidence has shown that it is the most sensitive single test, not only in primary syphilis but also in all stages of infection. The performance of TPPA is better than TPHA that may be partially attributable to greater binding of IgM by soluble antigens, which adhere to the gelatin particles better than to erythrocytes. The conventional testing algorithm of using both VDRL and TPHA as the screening tool may change as more evidence is available on the performance of TPPA in future.
Enzyme immunoassay (EIA) is a sensitive test that is now adopted by Public Health Laboratory Centre, Department of Health as the screening test. It can however be adopted as the confirmatory test if the other serological test is otherwise used in initial screening.
Cerebrospinal fluid
Neurosyphilis is characterised by:
pleocytosis: lymphocytes >5/μL. However, pleocytosis can be present in HIV mono-infection, making CSF result difficult for interpretation.
increased protein >0.4 g/L
positive CSF VDRL, but the test is negative in more than a quarter of neurosyphilis cases. Other corresponding syphilis serologic test, e.g. FTA-ABS on CSF may not be available in many laboratories.
Atypical presentations of syphilis in HIV/AIDS have been well reported in the literature. Some examples were: recurrent chancriform ulcer, seronegative secondary syphilis, early and accelerated progression to neurosyphilis, persistent high titred reactive reaginic test after apparently adequate treatment (serofast), relapse after apparently curative treatment, and treatment failure even after high dose intravenous penicillin. Lumbar puncture and cerebrospinal fluid (CSF) analysis is recommended for HIV infected people presenting with late latent syphilis or syphilis of unknown duration, high titre non-treponemal test e.g. >=1:32 or low CD4 count. There is controversy if lumbar puncture should also be performed in earlier stages such as in the secondary and early latent stages. Reactive VDRL-CSF is more specific whereas CSF FTA-Abs is more sensitive with respect to the diagnosis of neurosyphilis, i.e. positive VDRL-CSF in person with clinical suspicion of neurosyphilis confirms and a negative CSF FTA-Abs in an otherwise asymptomatic person excludes the diagnosis.
The treatment regimes for various stages of syphilis are the same as those non HIV-infected. However, penicillin based regimes are strongly recommended (except for weekly benzathine penicillin regime which only delivers low albeit prolonged blood level of drug but will not be able to achieve meaningful level in CSF). A pivotal study conducted in the US demonstrated that benzathine penicillin regime in HIV and early syphilis co-infected individuals had a serological relapse rate of 18% at six months. In people who are treated by these regimes, regular follow-up for relapse is essential. For people who are treated with procaine penicillin daily for 17 to 21 days, there are debates that they may not be required to have lumber puncture.22 For neurosyphilis, lumbar puncture should be repeated to monitor treatment response at 6 month interval till normalisation of CSF findings.
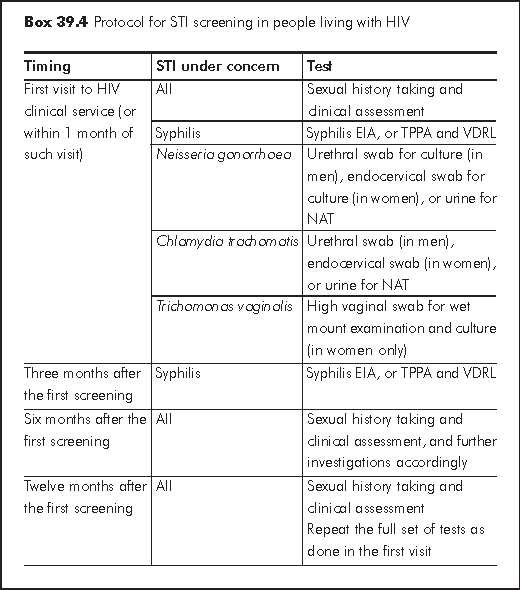
Some people living with HIV remain active sexually and hence are vulnerable to STI if safer sex practice is not consistently adopted. Recommendations on STI screening are developed in the US and UK with the objective of improving sexual health for people living with HIV. Such strategy carries also public health implication in the early detection of STI followed by not just treatment but appropriate behavioural interventions. In the process of implementing these recommendations, the service providers may need to strengthen their capacity in areas such as sexual health risk assessment and counselling, clinical and laboratory management of concerning STI, partner management, and sexual health referral network.
Screening strategies vary from one setting to another. Box 39.4 shows a protocol for use in HIV specialist clinic or primary care services.
Further tests for other STI or collection of clinical samples from sites other than those specified in Box 39.3 are indicated by results of clinical assessment. More frequent STI screening may be appropriate depending on individual risk behaviour, local STI epidemiology especially in an outbreak situation. The protocol is not meant to replace routine clinical management of people with symptomatology or clinical features suggestive of STI.
1. Centers for Disease Control and Prevention; Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11):1-94.
2. HIV prevention through early detection and treatment of other sexually transmitted diseases--United States. Recommendations of the Advisory Committee for HIV and STD prevention. MMWR Recomm Rep 1998;47(RR-12):1-24.
3. Nandwani R; Clinical Effectiveness Group of the British Association for Sexual Health and HIV. 2006 United Kingdom national guideline on the sexual health of people with HIV: sexually transmitted infections. Int J STD AIDS 2006;17:594-606.
4. Centre for Health Protection. Handbook of Dermatology and Venereology - Social Hygiene Handbook 3rd edition. Hong Kong: Department of Health, 2003.
Zhang KL, Ma SJ, Xia DY. Epidemiology of HIV and sexually transmitted infections in China. Sex Health 2004;1:39-46.
Centre for Health Protection. Social Hygiene Manual. Hong Kong: Department of Health, 2006.
WHO. Guidelines for the management of sexually transmitted infections. Geneva: WHO, 1999. WHO/RHR/01.10.
Ryan CA, Courtois BN, Hawes SE, Stevens CE, Eschenbach DA, Holmes KK. Risk assessment, symptoms, and signs as predictors of vulvovaginal and cervical infections in an urban US STD clinic: implications for use of STD algorithms. Sex Transm Infect 1998;74 Suppl 1:S59-76.
Grosskurth H, Mosha F, Todd J, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet 1995;346:530-6.
Grosskurth H, Gray R, Hayes R, Mabey D, Wawer M. Control of sexually transmitted diseases for HIV-1 prevention: understanding the implications of the Mwanza and Rakai trials. Lancet 2000;355:1981-7.
Kamali A, Quigley M, Nakiyingi J, et al. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: a community randomised trial. Lancet 2003;361:645-52.
White RG, Orroth KK, Korenromp EL, et al. Can population differences explain the contrasting results of the Mwanza, Rakai, and Masaka HIV/sexually transmitted disease intervention trials?: A modeling study. J Acquir Immune Defic Syndr 2004;37:1500-13.
Centre for Health Protection. Handbook of Dermatology and Venereology - Social Hygiene Handbook 3rd edition, Hong Kong: Department of Health, 2003, pp 453-82.
Tse C, Wong K, Fong O, Yeung W, Chan W, Yam W. Prevalence of asymptomatic Chlamydia trachomatis gonorrhoea urethritis among HIV-infected patients in Hong Kong. XVI International AIDS Conference, Toronto, Canada, 13-18 August 2006. [C43, TUPE0588].
Le Goff J, Weiss H, Grésenguet G, et al. Increased genital shedding of HIV-1 RNA in HSV-2- and HIV-1-co-infected African women with genital ulcer. Program and abstracts of the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16-19, 2005; Washington, DC. Abstract H-1500.
Ellerbrock TV, Chiasson MA, Bush TJ, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA 2000;283:1031-7.
Moscicki AB, Ellenberg JH, Farhat S, Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis 2004;190:37-45.
Young TP, Roark R, Cohan D, et al. CD4+ T-cell and HIV viral load associations with abnormal cervical and anal PAP smears in HIV-infected women. Program and abstracts of the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16-19, 2005; Washington, DC. Abstract H-1491.
Frisch M, Smith E, Grulich A, Johansen C. Cancer in a population-based cohort of men and women in registered homosexual partnerships. Am J Epidemiol 2003;157:966-72.
Greenberg LJ, Kouides RW, Corales RB. Risk factors for abnormal anal PAP smear conversion in HIV+ patients. Program and abstracts of the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; December 16-19, 2005; Washington, DC. Abstract H-1490.
Danso D, Lyons F, Bradbeer C. Cervical screening and management of cervical intraepithelial neoplasia in HIV-positive women. Int J STD AIDS 2006;17:579-87.
Tse CT, Hau KL, Ho KK, Ho KM. Early syphilis in people with HIV infection. Abstract bookof The HK Society for Infectious Diseases, Sixth Annual Scientific Meeting 23 March 2002, HKSAR, pp. 13.
Centers for Disease Control and Prevention. Tracking the Hidden Epidemics 2000 - a Closer Look at Syphilis. Atlanta: CDC, 2000. Available from www.cdc.gov/std/Trends2000/syphilis-close.htm (accessed 3 November 2006).
Leung WL, Kam KM. Use of enzyme immunoassay as treponemal screening test in syphilis diagnosis. HK J Dermatol Venereol 2006;14:189-95.
WHO. World Health Organization Treponemal Infections Technical reports series 674, 1982.
Nandwani R, Fisher M; Medical Society for the Study of Venereal Diseases HIV Special Interest Group. Clinical standards for the screening and management of acquired syphilis in HIV-positive adults. Int J STD AIDS 2006;17:588-93.