June SM LAU, Patrick CK LI
HIV-associated lymphomas are predominantly aggressive B-cell lymphomas. According to the World Health Organization (WHO) classification, HIV-associated systemic non-Hodgkin's lymphomas (HIV-NHLs) include diffuse large B-cell lymphoma (DLBCL), HIV-associated primary central nervous system lymphoma (HIV-PCNSL), Burkitt's lymphoma (BL), primary effusion lymphoma (PEL) and plasmablastic lymphoma of the oral cavity. Hodgkin disease, though not an AIDS-defining condition, is also increased in HIV-infected individuals. The epidemiology of HIV-associated lymphoma has changed since highly active antiretroviral therapy (HAART) becomes available in clinical practice.
In Hong Kong, 20 HIV-NHL patients have been managed at Queen Elizabeth Hospital from 1995 to 2006, providing information for updating those in a previous series.1 In this series, 17 patients had systemic NHLs and 3 PCNSLs. All PCNSLs were of B-cell origin. Among the systemic NHLs, 15 patients had aggressive B-cell lymphoma (DLBCL: 12, BL: 2, mantle cell lymphoma: 1) and 2 patients had plasmablastic lymphoma. The median CD4 count of patients with HIV-NHL was 60/μL. Among the patients with systemic NHL, use of HAART and having <=2 adverse factors on presentation according to International Prognostic Index (IPI) for aggressive NHL (stage III or IV disease, age >60 years, elevated lactate dehydrogenase, ECOG (Eastern Cooperative Oncology group) performance status >=2, >=2 extranodal sites) were associated with longer survival (Lau, unpublished data - Blood (ASH Annual Meeting Abstracts) 2006; 108: Abstract 3863).2
NHLs occur 160 times more frequently in HIV-infected patients as compared with the general population. Patients with HIV-NHL usually present with advanced stage disease and more frequently have B symptoms (fever, night sweats and weight loss) and involvement of uncommon sites, including the oral cavity, gastrointestinal tract and central nervous system. They are associated with Epstein-Barr virus (EBV) or human herpesvirus type 8 (HHV-8) infection. A decade into the HAART era, multiple studies have demonstrated the decrease in incidence and mortality of HIV-NHL.3,4
The diagnosis of HIV-NHL is normally established by histological confirmation through fine-needle aspiration, excisional biopsy of enlarged lymph nodes, or bone marrow biopsy. Staging is based on clinical examination, chest radiograph, computed tomographic (CT) examination of the abdomen and pelvis for lymphadenopathy or visceral involvement, and bilateral iliac crest trephine biopsy. Tests for blood counts, CD4 lymphocytes, renal and liver function, lactate dehydrogenase, calcium and uric acid level provide information of prognostic and management significance. Before HAART, the prognosis for HIV-infected individuals with NHL was poor with a median survival of 5-8 months.
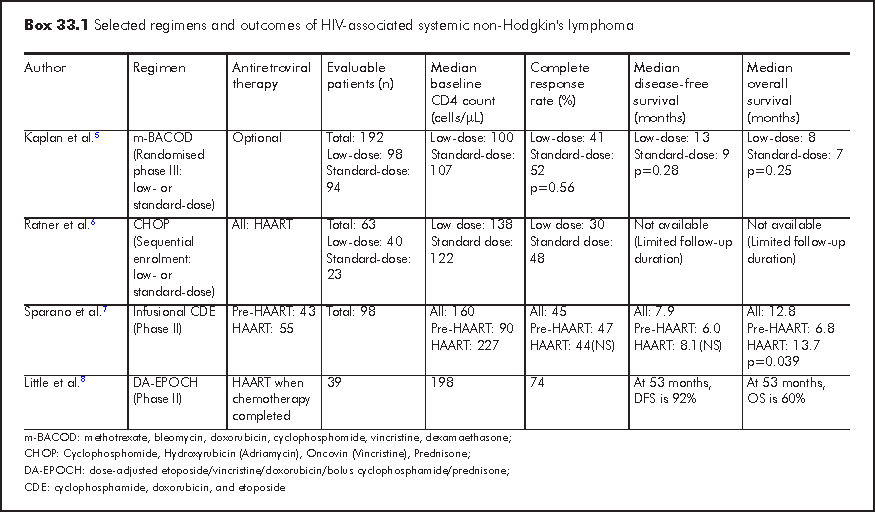
Before the widespread use of HAART, the AIDS Clinical Trials Group reported comparable response rate and overall survival in patients with HIV-NHL receiving low-dose versus standard-dose m-BACOD [methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, dexamethasone].5 The median survival in both groups was short (35 weeks in low-dose group versus 31 weeks in standard-dose group, p=0.25). Growth factor support was used routinely in the standard-dose group and administered as required in the low-dose group. There was lower incidence of grade 4 neutropaenia in the low-dose group (50% versus 69%, p=0.007). The result of this trial formed the basis for using low-dose chemotherapy in HIV-NHL during the pre-HAART era.
Immune restoration in response to HAART results in decreased incidence of opportunistic infections and better tolerance of chemotherapy. This facilitates the use of standard- or even high-dose chemotherapy in treating patients with HIV-NHL. In addition, infusional regimen has been developed to enhance the efficacy of the chemotherapeutic agents. The optimal treatment for HIV-associated systemic NHL has not been defined (Box 33.1). CHOP (Cyclophosphomide, Adriamycin, Vincristine, Prednisone) or DA-EPOCH (dose-adjusted etoposide/vincristine/doxorubicin/bolus cyclophosphamide/prednisone) is currently favoured in treating patients with HIV-NHL. Central nervous system prophylaxis with intrathecal chemotherapy should be considered for patients with risk factors for meningeal recurrence: small non-cleaved cell histology, bone marrow, paranasal sinus or testicular involvement or presence of epidural disease. Prophylaxis for opportunistic infection should be given in accordance to the CD4 count. Rituximab, if administered, should be used with great caution in patients with HIV-NHL, especially when the CD4 count is less than 50/μL because of the increased risk of life-threatening infectious complications.
Poor treatment response is associated with older age, advanced lymphoma staging or extranodal involvement, CD4 count below 100/μL or previous AIDS diagnosis and intravenous drug use. In addition, HIV-NHL has a higher incidence of multidrug resistance (MDR-1) expression which is associated with poorer response to conventional combination chemotherapy.

HIV-related primary central nervous system lymphoma (PCNSL) typically occurs in patients with CD4 count below 50/μL. Its incidence has decreased by three-folds since the introduction of HAART. The presenting signs and symptoms include headache, subtle personality changes, altered conscious level, memory loss, focal neurological deficit or seizures.
It is often difficult to distinguish PCNSL from cerebral lesions due to infection such as toxoplasmosis since both conditions can present as single or multiple contrast-enhancing mass lesions on CT or MRI. However, toxoplasmosis typically have ring-enhancing lesions and the surrounding oedema is not as marked as lymphoma.
Definitive histopathologic diagnosis of focal brain lesion in AIDS patients requires stereotactic biopsy. However, the location of some lesions may be technically inaccessible. In addition, it should be recognised that corticosteroid administered to reduce cerebral oedema can produce brisk tumour response, resulting in a false-negative biopsy. Hence, if brain biopsy is to be performed, it is advisable to withhold corticosteroid unless the patient is in immediate danger of herniation. Alternatively biopsy should be performed as soon as possible after the first dose of steroid.
Recent advances in the diagnosis of HIV-PCNSL include the use of Thallium-201 single-photon emission computerised tomography (SPECT) and detection of EBV-DNA in cerebrospinal fluid (CSF).
Nearly all HIV-PCNSL are associated with EBV infection, and EBV is otherwise rarely detected in the CSF of HIV patients without PCNSL. SPECT have been demonstrated to facilitate differentiation of malignant from infectious brain lesions. The presence of increased uptake on SPECT together with positive EBV DNA in the CSF had 100% sensitivity and 100% positive predictive value for PCNSL.9 With positive result for both tests, radiotherapy for PCNSL can be started without the need for brain biopsy. Negative results for both tests had 100% negative predictive value and can confidently exclude the diagnosis of lymphoma. With discordant results, brain biopsy should be performed to establish the diagnosis. For patients in whom SPECT and PCR examination for EBV DNA is not available and brain biopsy is not feasible, empirical radiotherapy for PCNSL may be tried after failure to respond to a course of anti-toxoplasmosis therapy.
Before the HAART era, the median survival for untreated AIDS-related PCNSL was approximately 4 to 8 weeks. Use of radiotherapy had resulted in improvement in the performance status and slightly prolonged the median survival to 2 to 5 months. However, most patients ultimately died of opportunistic infections.
HAART has led to improvement in the survival of patients with AIDS-related PCNSL, particularly for those with good CD4 and virological response. However, with extended survival of the patients, late complications of radiotherapy such as leukoencephalopathy and radiation necrosis may become a concern. Immune recovery following HAART has increased the feasibility of using systemic chemotherapy in AIDS-related PCNSL. In a series of 15 patients with AIDS-related PCNSL, up to six cycles of high dose methotrexate (3 g/m2 IV Q14 days) with leucovorin rescue were administered.10 Whole-brain radiation therapy was reserved for cases with disease progression or relapse. Seven of the 15 patients achieved complete radiological response. The overall median survival was 10 months while the median survival for complete responders was 19 months. Side effects, including gastrointestinal upset and mucositis, were tolerable and did not result in dose modification or treatment delay.
Current data support initiation or optimisation of HAART upon diagnosis of HIV-PCNSL. For patients with relatively good performance status or reasonably controlled HIV replication at diagnosis of HIV-PCNSL, systemic high-dose methotrexate should be considered, while reserving whole-brain irradiation for disease progression or relapse and for those patients who have contraindications for systemic chemotherapy.
Primary effusion lymphoma (PEL) accounts for approximately 1-5% of HIV-associated lymphoma and is seen most frequently in advanced AIDS. Clinically, the disease is characterised by lack of nodal or extranodal solid tumours with malignant serous effusions being the predominant feature. Kaposi sarcoma-associated herpesvirus (KSHV, HHV-8) can be demonstrated in nearly 100% of tumour specimens and has been implicated in the pathogenesis of this uncommon entity. EBV is also detectable in the majority of cases, raising the possibility of a second hit model of lymphomagenesis. Morphologically, PEL cells are distinct. They resemble large cells of a null phenotype on immunohistochemical stains, but immunoglobulin gene rearrangements are usually positive, indicating a clonal B-cell origin.
Before the advent of HAART, patients with HIV-PEL had short overall survival, ranging from 3 to 6 months. Results from small studies in recent years suggest that antiretroviral therapy has a beneficial effect in the treatment of PEL.
Boulanger et al reported a series of 28 patients with HIV-PEL diagnosed between 1993 and 2003, a period that straddled the advent of HAART.11 As first-line treatment, 5 patients received interferon-alpha either alone or in combination with cidofovir, while 23 received various combination chemotherapy. Seven patients received second line treatment with interferon-alpha (n=2) or combination chemotherapy (n=5). The complete remission rate was 50%, median survival was 6.2 months and one-year overall survival was 39.3%. In multivariate analysis, performance status greater than 2 and absence of HAART before PEL diagnosis were found to be independent predictors for shorter survival. Another series reported 8 PEL patients with 5 receiving HAART in addition to a CHOP-like regimen.12 One patient was treated with HAART alone and two other patients received neither chemotherapy nor HAART. Three of the 7 assessable patients (1 on CHOP plus HAART, 1 on CEOP plus HAART and 1 on HAART alone) achieved complete remission, resulting in a complete remission rate of 42% and median survival of 6 months.
There is no standard treatment for this rare entity. However, it would be reasonable to treat patients having HIV-PEL with HAART in conjunction with combination chemotherapy that are used for systemic HIV-NHL.
Plasmablastic lymphoma occurs predominantly in patients with advanced HIV disease, although it has also been reported in HIV-negative individuals. It accounts for approximately 2.6% of all HIV-NHL. The tumour primarily occurs in the jaw and oral cavity but it can also involve a variety of other sites, such as gastrointestinal tract and lung. Morphologically, the malignant cells resemble plasmablasts, but carry a phenotype typical of mature plasma cells with high proliferative fraction and are EBV-associated. On flow cytometry, the cells are negative for B- and T-cell markers but positive for markers of plasma cell differentiation, including VS38c, CD79a and CD138, light chain epithelial membrane antigen (EMA) and immunoglobulin G. Ki-67 staining is typically quite high in 75-95%. The absence of a serum monoclonal gammopathy helps to distinguish this entity from multiple myeloma.
Data from the pre-HAART era showed that plasmablastic lymphoma carried a poor prognosis. Delecluse and coworkers reported that 8 out of 10 patients with available follow up died, nearly all within 12 months of diagnosis.13
The prognosis has improved since the advent of HAART. Teruya-Feldstein and associates reported 6 HIV-positive patients with plasmablastic lymphoma being treated with various regimens.14 All 6 patients received HAART (4 patients were previously on HAART while 2 patients were newly diagnosed with HIV infection and initiated on HAART at the time of diagnosis of lymphoma). Five of the 6 patients were alive with a median follow up duration of 17 months. Lester and coworkers reported 2 patients with concurrent diagnosis of plasmablastic lymphoma and HIV infection.15 Both had good immunological and virological response to HAART and their lymphoma was in complete remission 10 and 25 months after initial diagnosis.
Currently no standard treatment is recommended for this rare entity. HAART and systemic chemotherapy with CHOP or CODOX/M-IVAC (cyclophosphamide, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine) had been used in reported series.
HIV-associated Hodgkin Disease (HIV-HD) is not included among the Centers for Disease Control and Prevention (CDC) AIDS-defining illnesses. However, several groups had demonstrated that there was increased risk of HD among patients with AIDS.
Compared to HIV-negative cases, patients with HIV-HD tend to present at a younger age. It's commoner to have extranodal or bone marrow involvement, advanced stage disease, B symptoms (fever, night sweat, weight loss), while mediastinal adenopathy appeared less frequently. Histologically, there is a predominance of two unfavourable subtypes, namely mixed cellular and lymphocyte depleted. EBV is positive in 78-100% of patients with HIV-HD as compared to 15-48% of patients with non-HIV-related HD. The patients often present with a higher median CD4 count (more than 275/μL in reported series) than patients with HIV-associated diffuse large cell lymphoma.
In the pre-HAART era, the median survival for patients with HIV-HD was only 8-20 months. With the advent of HAART, there is significant improvement in the outcome. A Spanish group studied 45 patients with HIV-HD treated from 1987 to 2001.16 A substantial improvement in the estimated 4-year survival was observed in the HAART era (83% versus 13%, p=0.001). Gerald and associates in France retrospectively reviewed 108 patients treated over a 15-year follow-up period.17 There was significant difference in estimated 2-year survival (45% in the pre-HAART period versus 62% in the HAART period, p=0.03), mainly due to a decrease in AIDS-associated deaths. The median survival was only 19 months in the pre-HAART period while the median survival time had not been reached at time of analysis for patients treated in the HAART era.
The optimal treatment regimen for HIV-HD has not been defined. Regimen that had been used in HIV-HD includes:
(a) ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine)
(b) EBVP (epirubicin, bleomycin, vinblastine, and prednisone)
(c) Standford V (doxorubicin, vinblastine, mecloretamine, etoposide, vincristine, bleomycin, prednisone)
(d) BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone)
Mak YK, Chan CH, Li CK, Lee MP, Tsang YW. Clinical profiles of human immunodeficiency virus-associated lymphoma in Hong Kong. Hong Kong Med J 2003;9:91-7.
Lau SMJ, Lee MP, Chan CH, Li CKP. Prognostic Factors for HIV-Associated Lymphoma - Experience in a Tertiary Referral Center in Hong Kong. Blood (ASH Annual Meeting Abstracts) 2006; 108: Abstract 3863.
Thirlwell C, Sarker D, Stebbing J, Bower M. Acquired immunodeficiency syndrome-related lymphoma in the era of highly active antiretroviral therapy. Clin Lymphoma 2003;4:86-92.
Palmieri C, Treibel T, Large O, Bower M. AIDS-related non-Hodgkin's lymphoma in the first decade of highly active antiretroviral therapy. QJM 2006;99:811-26.
Kaplan LD, Straus DJ, Testa MA, et al. Low-dose compared with standard-dose m-BACOD chemotherapy for non-Hodgkin's lymphoma associated with human immunodeficiency virus infection. National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group. N Engl J Med 1997;336:1641-8.
Ratner L, Lee J, Tang S, et al. Chemotherapy for human immunodeficiency virus-associated non-Hodgkin's lymphoma in combination with highly active antiretroviral therapy. J Clin Oncol 2001;19:2171-8.
Sparano JA, Lee S, Chen MG, et al. Phase II trial of infusional cyclophosphamide, doxorubicin, and etoposide in patients with HIV-associated non-Hodgkin's lymphoma: an Eastern Cooperative Oncology Group Trial (E1494). J Clin Oncol 2004;22:1491-500.
Little RF, Pittaluga S, Grant N, et al. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood 2003;101:4653-9.
Antinori A, De Rossi G, Ammassari A, et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol 1999;17:554-60.
Jacomet C, Girard PM, Lebrette MG, Farese VL, Monfort L, Rozenbaum W. Intravenous methotrexate for primary central nervous system non-Hodgkin's lymphoma in AIDS. AIDS 1997;11:1725-30.
Boulanger E, Gerard L, Gabarre J, et al. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J Clin Oncol 2005;23:4372-80.
Simonelli C, Spina M, Cinelli R, et al. Clinical features and outcome of primary effusion lymphoma in HIV-infected patients: a single-institution study. J Clin Oncol 2003;21:3948-54.
Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood 1997;89:1413-20.
Teruya-Feldstein J, Chiao E, Filippa DA, et al. CD20-negative large-cell lymphoma with plasmablastic features: a clinically heterogenous spectrum in both HIV-positive and -negative patients. Ann Oncol 2004;15:1673-9.
Lester R, Li C, Phillips P, et al. Improved outcome of human immunodeficiency virus-associated plasmablastic lymphoma of the oral cavity in the era of highly active antiretroviral therapy: a report of two cases. Leuk Lymphoma 2004;45:1881-5.
Ribera JM, Navarro JT, Oriol A, et al. Prognostic impact of highly active antiretroviral therapy in HIV-related Hodgkin's disease. AIDS 2002;16:1973-6.
Gerard L, Galicier L, Boulanger E, et al. Improved survival in HIV-related Hodgkin's lymphoma since the introduction of highly active antiretroviral therapy. AIDS 2003;17:81-7.