Ian CT TSE
Kaposi's sarcoma (KS) was the most common HIV-related malignancy. In the pre-HAART era, occurrence of KS among AIDS patients in the United States was over 20,000 times that in the general population and over 300 times commoner than other immunosuppressed patients such as renal transplant recipients.1 Early in the AIDS epidemic, it was noted that men having sex with men (MSM) had a higher chance of developing KS than those who had acquired HIV from other routes such as infusion of contaminated blood products. This finding led to the hypothesis that HIV-associated KS is caused by a sexually transmitted factor which was subsequently determined to be human herpesvirus 8 (HHV-8) or KS-associated herpesvirus (KSHV).2
HHV-8 is a transmissible DNA virus. MSM has a higher seroprevalence of HHV-8 infection than the general population. This virus is a necessary, but not sufficient cause of KS. It encodes viral IL-6 that leads to the expression of vascular endothelial growth factor and inflammatory-angiogenetic environment. The synergy of HIV-1 tat protein and immunosuppression explains why AIDS-related KS is more aggressive than the classic Mediterranean form in which the HIV-1 tat protein is not involved. The pathogenesis of AIDS-related KS is multifactorial and involves, not only HHV-8 but also (a) altered expression and response to cytokines, and (b) stimulation of KS growth by HIV-1 tat protein.
The overall incidence of HIV-associated KS has declined since the advent of effective antiretroviral therapy. In Hong Kong, KS accounted for 13% of AIDS-defining illness from 1985 to 1994.3 This proportion dropped substantially to 4% in the subsequent two years. No cases of KS were reported in the HAART era from 1988 to 2000.4
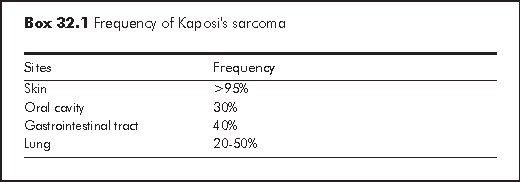
Although KS has been identified in virtually every site at autopsy, KS most frequently involves the skin. The most common extracutaneous sites are oral cavity, gastrointestinal tract and lung (Box 32.1).5 Diagnosis of KS is usually clinical, through the identification of visible lesions or endoscopic investigation of internal viscera lesions.

Typical KS skin lesions are violaceous macules, papules, plaques or nodules. Although the lesion is neither pruritic nor painful, it may be disfiguring as face is not an uncommon site of involvement. Other predisposed sites are lower extremities and genitalia. Lesions are often symmetrical. They may follow the skin lines giving rise to "Christmas tree pattern" on the back. Differential diagnoses of cutaenous KS include pyogenic granuloma, bacillary angiomatosis, angioma, melanocytic naevus, dermatofibroma or lichen planus. Secondary lymphoedema may develop due to obstruction of dermal lymphatics. It is most commonly observed in the lower extremities, but can also appear in the external genitalia or periorbital soft tissues.
All sub-types of KS (Classic type, African type, KS associated with immunosuppressive therapy and AIDS-related KS) have the same microscopic appearance. The earliest skin lesion (macule or patch) is predominantly vascular in nature. Within the dermis, there are dilated irregular vascular channels surrounding pre-existing vessel ("promontory sign"), extravasated erythrocytes and deposits of hemosiderin.6
The more advanced lesion (papules, nodules and plaques) is characterised by poorly defined slit-like vessels, dermal proliferation of spindle cells and mixed inflammatory cell infiltrates (lymphocytes and plasma cells).
Oral cavity KS may be the first manifestation of HIV infection. The most common site of involvement is hard palate, followed by gingiva. Other potential sites of involvement are soft palate, tongue, tonsils, floor of the mouth and pharynx. While most oral cavity disease is asymptomatic, it may be bulky or ulcerated and affect oral intake or speech.
KS may occur anywhere in the gastrointestinal tract. Although it is asymptomatic in most cases, it may be complicated by bleeding, pain or obstruction. Endoscopy is the investigation of choice to identify the red haemorrhagic nodule. Endoscopic biopsy may be negative due to submucosal location of the lesions.
Pulmonary KS without mucocutaneous disease occurs in 20% of patients.7 The symptoms and radiologic appearance of pulmonary KS are nonspecific and often indistinguishable from other opportunistic infections. Chest X-ray may show nodule, infiltrates, effusion or mediastinal lymphadenopathy. Bronchoscopy, which shows the characteristic erythematous submucosal plaques, is frequently necessary to establish the diagnosis.
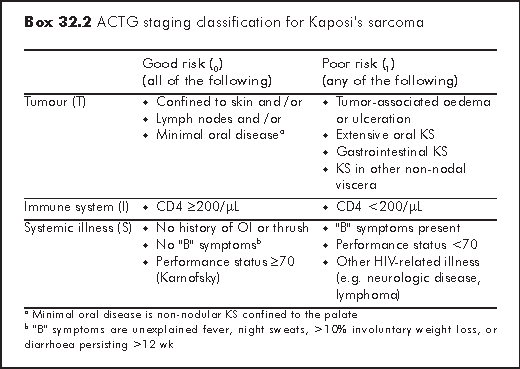
The tumour-node-metastases (TNM) classification used for most solid tumours is not suitable for staging KS, because the prognosis of KS does not only depend on the tumour per se but also on HIV disease status. AIDS Clinical Trials Group (ACTG) staging system is the most widely used staging system in KS (Box 32.2).8 Patients are classified as good or poor risk based on tumour burden (T), immune function (I) and severity of systemic illness (S). TIS staging predicts survival. Patients with only skin involvement, higher CD4 and who are free of HIV-related symptoms have better prognosis.
The major goals of treatment for KS are:
(a) to alleviate the symptoms
(b) to shrink tumours
(c) to prevent disease progression
KS treatment can be categorised into (a) HAART; (b) local therapy; and (c) systemic therapy (algorithm). Treatment options depend very much on the tumour behaviour (extent of disease and rate of growth), and the host status (CD4 count, psychosocial impact and other medical illnesses). Although KS is a systemic multi-focal disease, local therapies (Box 32.3) do offer significant palliation for many patients. Systemic therapy is indicated for:5
a) extensive tumour burden (>25 skin lesions)
b) visceral involvement
c) extensive oedema
d) "B" symptoms
e) treatment failure of local therapy
The choice and duration of using various modalities have to be titrated against response per treatment goals.
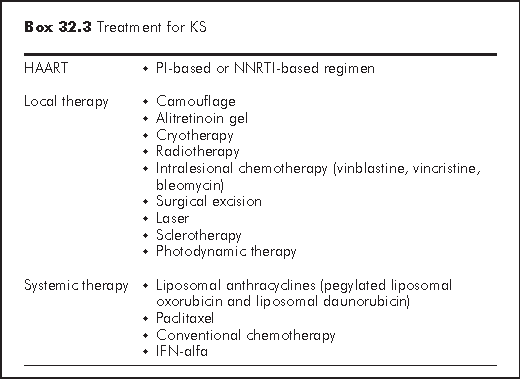
Reconstitution of the immune system with potent antiretroviral therapy has led to a decreased incidence, clinical improvement and even clearance of KS. HAART should be considered as the first-line treatment in HIV-infected patients who suffers from KS and have CD4 count <350/μL.9
Alitretinoin gel - Alitretinoin gel 0.1% (9-cis-retinoic acid) is a FDA-approved topical therapy for the treatment of KS. It is a naturally occurring retinoid that binds both to retinoic acid receptors (important in the regulation of cell proliferation and differentiation) and to retinoid X receptors (important in the regulation of apoptosis). It is applied twice daily on the cutaneous lesion. In a study of 268 patients with KS, clinical response was observed in 35% of patients treated with alitretinoin compared with 18% of patients treated with a vehicle gel.10 Clinical response was observed in most patients after 4 to 8 weeks of treatment. However, this gel does not prevent the development of new lesions in untreated areas. Mild to moderate cutaneous irritation on the site of application is also common.
Cryotherapy - Cryotherapy with liquid nitrogen is a simple and yet useful palliative treatment for small cosmetically unacceptable lesions. Nevertheless, cryotherapy may cause blister, unsightly scarring, and hypopigmentation.
Radiation therapy - Radiation therapy (usually low dose, 400 rads/week x 6 weeks) offers palliation for peri-orbital oedema, disfiguring lesions, obstructing lesions of the extremities, anus or rectum and painful disease. The treatment is usually well tolerated, but mucositis and opportunistic infections are common after radiation of the oral lesions.
Intralesional therapy - Intralesional injections of vinblastine (0.1 mL/0.5 cm2 at concentration of 0.2 mg/mL) have been used to treat solitary lesions. The response is usually partial. Tumour regrowth is common. The toxicity of intralesional therapy includes pain, hyperpigmentation, intense oedema and erythema.
Liposomal anthracyclines - Compared with nonliposomal chemotherapy, the liposomal formulations of the anthracyclines provide the theoretical advantage of longer plasma half-life, higher drug concentration in the tumour and fewer toxic effects in non-target organs. The two currently approved liposomal anthracyclines are pegylated liposomal doxorubicin (10-40 mg/m2 every 2-3 weeks) and liposomal daunorubicin (40-60 mg/m2 every 2 weeks). Both are considered as first-line systemic therapy for KS because of their good clinical response and relatively few side effects. These two drugs have similar toxicity including dose-related myelotoxicity, nausea, vomiting, alopecia, acute infusion-related reactions and palmar-plantar erythrodyaesthesia.11
Paclitaxel - Paclitaxel is an active agent against KS with efficacy similar to anthracyclines. It should be administered as a 3-hour infusion at a dose of 135 mg/m2 every 3 weeks. A lower dose of 100 mg/m2 two-weekly also appears to preserve efficacy and reduce toxicity.12 Potential side effects are plentiful, including neutropaenia, thrombocytopaenia, type 1 hypersensitivity reactions (bronchospasm, urticaria, & hypotension), sensory peripheral neuropathy, myalgia, arrhythmia and alopecia.
Conventional chemotherapy - The commonly used conventional chemotherapy is adriamycin, bleomycin plus vinblastine; bleomycin plus vinca alkaloids or vincristine/vinblastine (alone).
Interferon alfa - Interferon alfa is an attractive therapeutic option due to its known antiviral, antiproliferative, antiangiogenic and immune-modulating effects. Efficacy has been established on modest disease. The dose is 8,000 units SC/day. However the drug-related toxicity limits its usefulness.
Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi's sarcoma among persons with AIDS: a sexually transmitted infection? Lancet 1990;335:123-8.
Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 1994;266:1865-9.
Chan LY, Lee SS, Wong KH, Ng KK, Li PCK. KS in patients with AIDS the HK experience. HKMJ 1996;2:127-31.
Hong Kong Department of Health. An overview of HIV/AIDS situation in Hong Kong. Hong Kong STD/AIDS Update 2001;7(1):6-19.
Barlett J, Gallant JE. Medical Management of HIV Infection, 2004.
Weedon D. Skin pathology. Second Edition. Churchill Livingstone, 2002.
Cadranel JL, Kammoun S, Chevret S, et al. Results of chemotherapy in 30 AIDS patients with symptomatic pulmonary Kaposi's sarcoma. Thorax 1994;49:958-60.
Krown SE, Testa MA, Huang J. AIDS-related Kaposi's sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 1997;15:3085-92.
Wilkins K, Turner R, Dolev JC, LeBoit PE, Berger TG, Maurer TA. Cutaneous malignancy and human immunodeficiency virus disease. J Am Acad Dermatol 2006;54:189-206.
Walmsley S, Northfelt DW, Melosky B, Conant M, Friedman-Kien AE, Wagner B. Treatment of AIDS-related cutaneous Kaposi's sarcoma with topical alitretinoin (9-cis-retinoic acid) gel. Panretin Gel North American Study Group. J Acquir Immune Defic Syndr 1999;22:235-46.
Powderly WG. Manual of HIV therapeutic. Philadelphia. Lippincott-Raven, 1997.
Tulpule A, Groopman J, Saville MW, et al. Multicenter trial of low-dose paclitaxel in patients with advanced AIDS-related Kaposi sarcoma. Cancer 2002;95:147-54.