Man-Po LEE
Pneumocystis organisms were first reported by Chagas in 1909. It was first thought to be a protozoan but was reclassified as a fungus in 1988 after DNA sequence analysis. More recently, additional DNA data showed that Pneumocystis organisms from different host species have very different DNA sequences, indicating multiple species. The microbe that causes human PCP is now named Pneumocystis jiroveci.1 However changing the organism's name does not preclude the use of the acronym PCP because it can be read "Pneumocystis pneumonia".
Before the widespread use of chemoprophylaxis, PCP was a major cause of morbidity and mortality in AIDS patients. Some 30-60% of untreated AIDS patients would have PCP at the time of the AIDS diagnosis and an additional 20-35% develop PCP sometime after the AIDS diagnosis.2 Incidence of PCP has declined substantially in the HAART era; the incidence of which has fallen from 4.9 cases per 100 person-years before March 1995 to 0.3 cases per 100 person-years after March 1998 in industrialised countries. A majority of cases now occur among patients who are unaware of their HIV infection or are not receiving ongoing HIV care.3,4 PCP is the most common AIDS-defining illness in Hong Kong.
The environmental source of pneumocystis remains uncertain and the transmission is not fully understood. Studies support the theory of infection early in life and reactivation of latent infection when patients become immunocompromised. There is recent evidence of person-to-person transmission as the mode of acquiring new infections.5 Although the results of studies in animals and humans favour airborne transmission, respiratory isolation for patients with PCP is not currently recommended.
PCP is often the AIDS-defining illness in HIV-infected patients, usually occurring when CD4 count drops below 200 cells/μL. Common symptoms include subacute onset of dyspnoea, nonproductive cough, and low-grade fever. Acute dyspnoea with pleuritic chest pain may indicate the development of pneumothorax. Physical examination typically reveals tachypnoea, tachycardia, and normal findings on lung auscultation. Extra-pulmonary involvement can rarely occur particularly in patients on aerosolised pentamidine.
Typical radiographic features of PCP are bilateral perihilar interstitial infiltrates that become increasingly homogeneous and diffuse as the disease progresses. Less common findings include solitary or multiple nodules, upper-lobe infiltrates in patients receiving aerosolised pentamidine, pneumatoceles, and pneumothorax. Pleural effusions and thoracic lymphadenopathy are rare. High-resolution computed tomography is more sensitive than chest radiography and may reveal extensive ground-glass attenuation or cystic lesions.
PCP may be difficult to diagnose from nonspecific symptoms and signs, and that there may be co-infection with other organisms (such as CMV) in the immunocompromised host. Pneumocystis cannot be cultured, and thus diagnosis of PCP requires microscopic examination of sputum, bronchoalveolar fluid or lung tissue.6
Sputum induction with hypertonic saline has a diagnostic yield of 50 to 90% and should be the initial procedure used. If it fails to make a diagnosis, bronchoscopy with bronchoalveolar lavage should be performed. Trophic forms can be detected with Wright-Giemsa or Papanicolaou stain. Cysts can be stained with methenamine silver stain. Direct and indirect immunofluorescent methods have been shown to have greater sensitivity than the classical staining methods.
The use of the polymerase chain reaction (PCR) to detect pneumocystis nucleic acids has been an active area of research. PCR has been shown to have greater sensitivity and specificity for the diagnosis of PCP from specimens of induced sputum and bronchoalveolar lavage fluid than conventional staining.
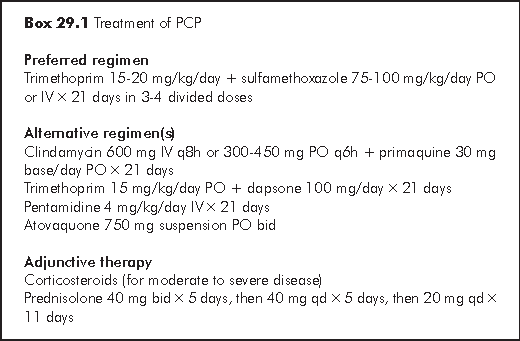
Trimethoprim-sulfamethoxazole (TMP-SMZ) is the golden standard for the treatment of PCP and extrapulmonary disease. It has the advantage of excellent penetration, rapid clinical response (3 to 5 days in patients with mild to moderate disease), and good oral bioavailability. TMP-SMZ is effective in treating PCP of different severities. Unfortunately, adverse events are common in HIV-infected patients due to systemic glutathione deficiency. The adverse effects of TMP-SMZ are generally those of sulfa drug: skin rash including Stevens-Johnson syndrome, fever, transaminase level elevation, neutropaenia, thrombocytopaenia, and nephrotoxicity.

Pentamidine has been the main alternative parenteral agent for the treatment of PCP. It achieves therapeutic levels in the lungs slowly (5 to 7 days) due to high levels of extrapulmonary tissue binding. Slow accumulation of pentamidine in pulmonary tissue may account for the delayed onset of activity when compared with TMP-SMZ. Pentamidine is useful for infection in patients with adverse reactions to TMP or sulfonamides. Side effects include transient hypoglycaemia, pancreatitis, diabetes, pancytopaenia, hypotension, and renal dysfunction. These side effects occur more frequently in patients with existing renal impairment. Renal function, electrolytes and blood glucose should be monitored closely during therapy.
Clindamycin-primaquine. The combination of clindamycin and primaquine is effective in patients with mild to moderate infection. No significant differences were observed among treatment groups consisting of AIDS patients receiving TMP-SMZ or clindamycin-primaquine for mild to moderate PCP in terms of survival, toxicity or treatment failure.7 The main toxicities of clindamycin include rash (16%), methaemoglobinaemia, anaemia, neutropaenia, and the development of Clostridium difficile colitis.
Dapsone-trimethoprim. Dapsone in combination with TMP is an effective oral therapy for mild to moderate PCP. The efficacy of dapsone-trimethoprim is similar to that of TMP-SMZ, but dapsone-trimethoprim was associated with a lower frequency of major toxicities (30% compared with 57%).8 In a subsequent randomised trial, however, the rates of dose-limiting toxicity among patients receiveing these regimens were similar.7 Toxicities include neutropaenia, anaemia, fever, haemolysis in patients with G6PD deficiency, rash and hepatitis.
Atovaquone is approved by the US FDA for the treatment of mild to moderately severe PCP. Side effects are relatively uncommon and are generally mild. Up to 7% of HIV-infected patients develop dose-limiting toxicity while on atovaquone (versus 20% for those on TMP-SMZ therapy); however, significantly more patients in the atovaquone group than in the TMP-SMZ group failed therapy. Toxic effects include rash, diarrhoea, nausea, vomiting, fever, and deranged liver function.
Studies have shown that the use of corticosteroids can prevent deterioration in oxygenation, intubation and mortality (50% reduction). Steroid therapy is recommended in patients with moderate-to-severe PCP. Criteria include a partial arterial O2 pressure (PaO2) of less than 70 mmHg in room air or an alveolar-arterial oxygen gradient of greater than 35 mmHg. Gradual tapering of dosage (usually over 2 weeks) is necessary to avoid relapse of pulmonary inflammation. Adverse drug reactions and complications of steroid therapy have been relatively rare.
Response to therapy is generally excellent in patients whose infection is diagnosed prior to respiratory failure. Failure to observe clinical improvement by day 4 to 5 (for those receiving TMP-SMZ) or days 5 to 7 (for those receiving pentamidine) should warrant a search for other pathology. The optimal duration of therapy has not been studied, but it is generally given for 14 to 21 days. Residual viable organisms may persist after treatment for up to a year.
Treatment failure with a recommended regimen is uncommon. While some patients may do better on one agent instead of another, it is commoner to discover a second process (infection, tumour, allergy, adult respiratory distress syndrome) complicating PCP than resistance that has emerged. The chest radiograph is less reliable than the level of arterial blood oxygenation as an indicator of treatment failure.
Immune reconstitution disease can occur in patients with PCP though only limited data are available. There were case reports of transient clinical deterioration in patients with confirmed PCP soon after the introduction of HAART (3 to 17 days).9,10 In these cases, patients initially responded to PCP treatment but developed acute respiratory failure shortly after the initiation of antiretroviral therapy. They normally recovered after reintroduction of systemic steroid.
Indications. HIV-infected patients should receive primary prophylaxis against PCP if they have a CD4+ T-lymphocyte count <200 cells/μL or a history of oropharyngeal candidiasis. Patients who have a history of PCP should be administered secondary prophylaxis (chronic maintenance therapy) for life unless immune reconstitution occurs as a result of ART.11
TMP-SMZ is the first choice for the prevention of PCP. A variety of prophylactic regimens have been studied. Studies of low- and high-dose regimens (single- or double-strength TMP-SMZ) for prophylaxis suggest no advantage in reducing mortality with the higher dose (12% incidence in the high-dose group versus 15% incidence in the lower-dose group). No new PCP was observed in either group when the patients were compliant with treatment. Intermittent therapy (double strength TMP-SMZ 3 times per week) has also been found to be equally effective.
Toxicities of TMP-SMZ generally occurred within the first month of chemoprophylaxis unless they are masked by immune suppression. AIDS patients with mild adverse effects often tolerate the reintroduction of the drugs at a lower dose after resolution of symptoms (such as rash). Rapid oral desensitisation is possible and is well tolerated in up to 86% of patients tested in small series.12
Alternative prophylactic regimens are available for patients intolerant of TMP-SMZ. Pentamidine aerosol prophylaxis is effective when it is administered by experienced personnel with a nebuliser (e.g. Fisons or Respirgard II) which produces droplets in the 1- to 3-mm range. Breakthrough infection is seen in a range of 10 to 23% of compliant patients, most often in those with rapidly progressive AIDS or CD4 counts of less than 50/μL. Because aerosolised drug may not reach the upper lobes, breakthrough infection of the upper lobes has often been observed. Side effects of aerosolised pentamidine are usually minimal. Cough and bronchospasm are common and are generally reversible with bronchodilator therapy.
Dapsone is another prophylactic agent. A meta-analysis of 35 randomised trials of PCP prophylaxis suggested that TMP-SMZ is superior to pentamidine and lower-dose dapsone (the equivalent of 25 mg/day) for prophylaxis but is equivalent to higher-dose dapsone regimens (50 to 100 mg/day).13 TMP-SMZ and dapsone have similar anti-Toxoplasma efficacies. The incidence of intolerance to dapsone is roughly equivalent to that to TMP-SMZ (65 to 70%). Up to 40% of the patients who discontinue prophylactic therapy with either of these agents due to toxicity will not be able to tolerate the other drug. Switching from TMP-SMZ to dapsone cannot be recommended for individuals with severe side effects including desquamation, neutropaenia, severe nephritis, or hepatitis or in patients with documented G6PD deficiency.
Discontinuation of prophylaxis. Primary and secondary prophylaxis can be discontinued in patients who have responded to HAART with an increase of CD4+ count to >200 cells/μL for at least 3 months. Studies have confirmed the safety of practice.14,15 Besides, discontinuation of prophylaxis can reduce pill burden, potential toxicity and interactions, and selection of drug-resistant pathogens. Prophylaxis should be reintroduced if CD4 count drops below 200 cells/μL.
Stringer JR, Beard CB, Miller RF, Wakefield AE. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerg Infect Dis 2002;8:891-6.
Lundgren JD, Barton SE, Lazzarin A, et al. Factors associated with the development of Pneumocystis carinii pneumonia in 5,025 European patients with AIDS. AIDS in Europe Study Group. Clin Infect Dis 1995;21:106-13.
Lundberg BE, Davidson AJ, Burman WJ. Epidemiology of Pneumocystis carinii pneumonia in an era of effective prophylaxis: the relative contribution of non-adherence and drug failure. AIDS 2000;14:2559-66.
Wolff AJ, O'Donnell AE. Pulmonary manifestations of HIV infection in the era of highly active antiretroviral therapy. Chest 2001;120:1888-93.
Morris A, Beard CB, Huang L. Update on the epidemiology and transmission of Pneumocystis carinii. Microbes Infect 2002;4:95-103.
Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004;350:2487-98.
Safrin S, Finkelstein DM, Feinberg J, et al. Comparison of three regimens for treatment of mild to moderate Pneumocystis carinii pneumonia in patients with AIDS. A double-blind, randomized, trial of oral trimethoprim-sulfamethoxazole, dapsone-trimethoprim, and clindamycin-primaquine. ACTG 108 Study Group. Ann Intern Med 1996;124:792-802.
Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med 1990;323:776-82.
Wislez M, Bergot E, Antoine M, et al. Acute respiratory failure following HAART introduction in patients treated for Pneumocystis carinii pneumonia. Am J Respir Crit Care Med 2001;164:847-51.
Dean GL, Williams DI, Churchill DR, Fisher MJ. Transient clinical deterioration in HIV patients with Pneumocystis carinii pneumonia after starting highly active antiretroviral therapy: another case of immune restoration inflammatory syndrome. Am J Respir Crit Care Med 2002;165:1670.
Kaplan JE, Masur H, Holmes KK; USPHS; Infectious Disease Society of America. Guidelines for preventing opportunistic infections among HIV-infected persons--2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. MMWR Recomm Rep 2002;51(RR-8):1-52.
Gluckstein D, Ruskin J. Rapid oral desensitization to trimethoprim-sulfamethoxazole (TMP-SMZ): use in prophylaxis for Pneumocystis carinii pneumonia in patients with AIDS who were previously intolerant to TMP-SMZ. Clin Infect Dis 1995;20:849-53.
Ioannidis JP, Cappelleri JC, Skolnik PR, Lau J, Sacks HS. A meta-analysis of the relative efficacy and toxicity of Pneumocystis carinii prophylactic regimens. Arch Intern Med 1996;156:177-88.
Furrer H, Egger M, Opravil M, et al. Discontinuation of primary prophylaxis against Pneumocystis carinii pneumonia in HIV-1-infected adults treated with combination antiretroviral therapy. Swiss HIV Cohort Study. N Engl J Med 1999;340:1301-6.
Ledergerber B, Mocroft A, Reiss P, et al. Discontinuation of secondary prophylaxis against Pneumocystis carinii pneumonia in patients with HIV infection who have a response to antiretroviral therapy. Eight European Study Groups. N Engl J Med 2001;344:168-74.