Ka-Hing WONG
Cryptococcus neoformans is a budding encapsulated solid yeast found ubiquitously in soil. There are four serotypes of C. neoformans divided into two groups: C. neoformans var. neoformans and C. neoformans var. gatti. Clinical diseases seen in HIV/AIDS patients are almost exclusively caused by C. neoformans var. neoformans, which is often serious and potentially life-threatening.
Though C. neoformans is commonly found in pigeon faeces, there is no evidence that exposure is associated with increased risk of cryptococcosis. It is believed that C. neoformans enters the human body via the respiratory tract. Elimination of C. neoformans is through cell-mediated immunity, with the participation of neutrophils, macrophages and cytotoxic T lymphocytes. In the face of immunodeficiency, control of the infection fails. The fungus may then disseminate to the central nervous system or other organs. However, the exact mechanism of dissemination remains unclear.
Cryptococcal disease occurs in both immunocompetent and immunocompromised people. In a study of 46 culture- or histology-proven cryptococcosis seen at 2 acute public hospitals in Hong Kong from 1995 to 2005, 9 (19.6%) were HIV positive.1 Cryptococcosis in HIV infected patients may manifest as symptomatic or asymptomatic pneumonia but the commonest presentation is still meningitis. Overseas data showed that some 6-10% of AIDS patients would develop cryptococcal meningitis.2,3 In 3022 AIDS patients with extrapulmonary cryptococcosis before the availability of effective HIV treatment, 80% had meningitis and their median survival was only 8.4 months.4 Cryptococcal meningitis has been the second commonest fungal primary AIDS-defining illness in Hong Kong, occurring in some 6% of the AIDS patients. It is the most frequent neurological presentation of AIDS, developing at a median CD4 count of 43/μL.5
Immunocompetent patients with cryptococcosis tend to present with localised, indolent neurological disease and better clinical outcomes.1 On the contrary, cryptococcosis is a major fungal infection in HIV/AIDS patients. Its clinical presentation can be subtle, ranging from non-specific features of fever, malaise, mild headache to, sometimes, nausea and vomiting. Neck stiffness is an infrequent sign. Severe cases can present with encephalopathic features such as personality change and confusion, which also carry a worsened prognosis.6 Dissemination of the infection is common in AIDS patients, to e.g. liver and lymph nodes. Skin lesions resemble that of molluscum contagiosum.
Pulmonary involvement, which may be isolated, is not uncommon at initial presentation. The classic symptoms are cough, sputum, dyspnoea, chest pain and fever and abnormal chest X-ray. Rapidly fatal cryptococcosis was reported in 19 AIDS patients showing features of acute respiratory distress syndrome.7
Though cryptococcal meningitis carries a sinister prognosis and occurs mostly in patients with low CD4 count, routine antifungal prophylaxis is not recommended. This is because of the lack of survival benefit with primary prophylaxis, potential for development of resistance, possibility of drug interactions and cost.8
Preliminary diagnosis of cryptococcal infection is made by identification of the yeast in a compatible clinical setting. Definitive diagnosis is confirmed by the culture of specimens, often the cerebrospinal fluid (CSF) or blood, and sometimes in respiratory secretions.
Serum cryptococcal antigen (CRAG) is an accurate and sensitive predictor of cryptococcal meningitis in advanced patients with fever. It has been shown that 98% of patients with culture-proven cryptococcal meningitis have a positive serum CRAG.3 A positive titer >1:8 should be taken as a presumptive diagnosis of cryptococcal meningitis. Blood culture may be positive (50-70%) in case of systemic infection.
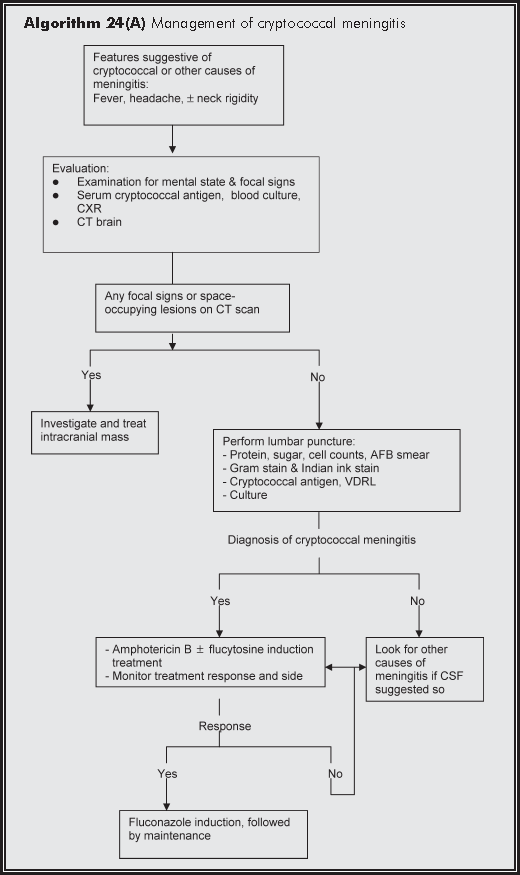
Lumbar puncture for CSF examination is indicated to diagnose or exclude meningitis, when the clinical picture is suggestive, or in the presence of extraneural disease or antigenaemia. Before the procedure CT scan must be performed to rule out space-occupying lesion, especially when there are focal neurological signs or encephalopathic changes suggestive of raised intracranial pressure. Opening pressure of CSF is to be recorded as this is of prognostic significance. Encapsulated yeast in the CSF may be detected with the Indian ink stain. CRAG and fungal culture results of CSF are useful for confirming the diagnosis subsequently.
CSF examination is important for both diagnosis and for predicting prognosis of cryptococcal meningitis. Unlike HIV negative patients, cryptococcal meningitis in AIDS patients can have normal protein/sugar and no pleocytosis in up to half of the cases.9 In one study, Indian ink stain of CSF revealed the organisms in 75% of the HIV-infected patients while fungal culture was positive in 85%.10 CRAG in CSF is very useful for diagnosis - positive in up to 95%. Several CSF parameters are associated with poor prognosis: a raised opening pressure, low white blood cell count, high antigen titer and more organisms.3
Treatment of cryptococcosis is indicated for patients with symptoms and all immunocompromised patients. Immunocompetent patients with confirmed infection but not yet disease should also be considered for therapy. The goal of treating cryptococcal pneumonia is to cure the lung infection and prevent disease dissemination, mainly to central nervous system. The primary objectives of treating cryptococcal meningitis are relief of symptoms and signs, control of infection, decrease in early mortality, prevention of relapse and maintenance of patient's quality of life. Prompt treatment with antifungals is especially important for patients with severe meningitis and poor prognostic factors. Treatment aims at bringing down the fungal burden in the CSF or blood to the point of sterile cultures.
Elevated intracranial pressure might cause clinical deterioration despite microbiological response. Repeated lumbar puncture is indicated for raised intracranial pressure, which also relieves symptoms. CSF shunting should be considered in patients failing or intolerant of daily lumbar puncture. Acetazolamide has no role for raised intracranial pressure.
Amphotericin B and fluconazole are the two drugs found to be most effective in treating AIDS-related cryptococcal meningitis.6 Flucytosine, though recommended for the induction phase,11 adds to the marrow toxicity of amphotericin B and such combination may not be well tolerated, especially in advanced disease patients.
High-dose amphotericin B, with or without 5-flucytosine, followed by fluconazole is the standard induction treatment for acute cryptococcal meningitis. Though the duration of intravenous amphotericin B induction treatment is arbitrary, it is generally given at dose of 0.5-0.7 mg/kg/day for 2 weeks, followed by consolidation therapy of fluconazole 200 mg bid for 8 weeks if response to treatment is good. This approach is associated with a mortality of <10% and a mycology response of 70%.12 The use of 5-flucytosine (100 mg/kg/day) during the first 2 weeks of primary therapy does not improve immediate outcome but may contribute to the prevention of relapse.13Combination fluconazole and flucytosine is also effective but more toxic. Cryptococcal pneumonia can be treated with fluconazole alone for both induction and maintenance. Resistance to Amphotericin B and azole drug is rare for cryptococcal diseases.
Response to acute antifungal treatment is monitored against clinical, biochemical and microbiological parameters. Toxicity of drugs should also be monitored. For example, fluconazole inhibits cytochrome P450 hepatic enzymes, thus increases the levels of drugs such as rifabutin, terfanadine, clarithromycin and cisapride. The risk of uveitis from rifabutin and cardiac arrhythmia from terfanadine and cisapride will be increased with concomitant fluconazole.
Complete blood picture, renal and liver function tests are performed at intervals no less than twice per week during amphotericin B therapy. Lipid formulations of amphotericin B are less toxic. The treatment response is best gauged by clinical assessment. Repeat examination of CSF may not be required in patients who responded well clinically.11 The median time for sterilising CSF is about 2 weeks for amphotericin B and more than 4 weeks for fluconazole.6 Serum CRAG cannot be used to monitor disease progress, treatment response or predict relapse of the infection.14 While serial measurement of CSF CRAG titer might be useful, it is not routinely recommended. Treatment failure is defined as the absence of clinical response in 2 weeks, or relapse after initial response. Lumbar puncture has to be repeated in such cases. Susceptibility testing of Cryptococcus neoformans is not standardised and not used in routine clinical practice.
Treatment options for failure are to: (a) continue amphotericin B for longer period, (b) give a higher dose of fluconazole, and (c) use alternative drugs like voriconazole or caspofungia though response to the latter drug is often suboptimal.
In the pre-HAART era, chronic suppressive treatment for life is required after primary treatment of cryptococcal meningitis as relapse rates of 50-60%15 and shortened survival have been found for patients not receiving such maintenance. Fluconazole at a dose of 200 mg per day is the preferred choice. Itraconazole is an alternative for maintenance therapy but is less effective than fluconazole.16 Amphotericin B maintenance is also inferior. A higher than usual (1.5-2 times) dose of fluconazole might be needed for consolidation and maintenance in some difficult cases. Despite maintenance, relapse should be watched out for, especially in patients with persistent severe immunosuppression. However, patients who completed primary treatment, remained asymptomatic and had sustained rise in CD4 count are at low risk of disease relapse.
There's significant reduction in the incidences of cryptococcosis in the post-HAART era. Lack of antiretroviral therapy and a prior HIV diagnosis were risk factors for development of the infection. Selected cohort studies provided support for the discontinuation of maintenance therapy for cryptococcal meningitis in patients with AIDS treated with HAART. One retrospective study of patients with CD4 >100/μL after HAART showed that recurrence was uncommon, which should be suspected when serum cryptococcal antigen reverts back to positive after discontinuation of maintenance therapy.17 In the EuroSIDA study of 358 patients with interruption of maintenance therapy of 4 major opportunistic infections, none of 39 patients with extrapulmonary cryptococcosis developed relapse.18 Patients who are discontinued on maintenance should have it reinitiated if CD4 decreases.
Immune reconstitution disease (IRD, refer to chapter 15) represents the undesirable effects of immune restoration in cryptococcosis patients after HAART. IRD-related cryptococcosis in patients with the first episode of infection and treated with HAART was observed more frequently in severely immunocompromised patients with disseminated infection and HAART initiation soon after the diagnosis.19 Latent cryptococcal infection can also be unmasked after HAART.20 Biochemically, IRD-related Cryptococcus neoformans may have higher cerebrospinal opening pressures, glucose levels and white cell counts when compared with typical HIV-associated cryptococcal meningitis.20
Lui G, Lee N, Ip M, et al. Cryptococcosis in apparently immunocompetent patients. QJM 2006;99:143-51.
Dismukes WE. Cryptococcal meningitis in patients with AIDS. J Infect Dis 1988;157:624-8.
Chuck SL, Sande MA. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med 1989;321:794-9.
Horsburgh CR, Selik RM: Extrapulmonary cryptococcosis in AIDS patients: Risk factors and association with decreased survival. [Abstract 564] 28th Interscience Conference on Antimicrobial Agents and Chemotherapy, 1988.
Wong KH, Lee SS. Comparing the first and second hundred AIDS cases in Hong Kong. Singapore Med J 1998;39:236-40.
Saag MS, Powderly WG, Cloud GA, et al. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med 1992;326:83-9.
Visnegarwala F, Graviss EA, Lacke CE, et al. Acute respiratory failure associated with cryptococcosis in patients with AIDS: analysis of predictive factors. Clin Infect Dis 1998;27:1231-7.
1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with Human Immunodeficiency Virus. USPHS/IDSA Prevention of Opportunistic Infections Working Group. Infectious Diseases Society of American. Ann Intern Med 1999;131:873-908.
Shaunak S, Schell WA, Perfect JR. Cryptococcal meningitis with normal cerebrospinal fluid. J Infect Dis 1989;160:912.
Zerpa R, Huicho L, Guillen A. Modified India ink preparation for Cryptococcus neoformans in cerebrospinal fluid specimens. J Clin Microbiol 1996;34:2290-1.
CDC. Treating opportunistic infections among HIV-infected adults and adolescents. Recomnmendations from the CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. MMWR Recomm Rep 2005;r53(RR-15):32-4.
van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med 1997;337:15-21.
Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis 2000;30:710-8.
Powderly WG, Cloud GA, Dismukes WE, Saag MS. Measurement of cryptococcal antigen in serum and cerebrospinal fluid: value in the management of AIDS-associated cryptococcal meningitis. Clin Infect Dis 1994;18:789-92.
Zuger A, Louie E, Holzman RS, Simberkoff MS, Rahal JJ. Cryptococcal disease in patients with the acquired immunodeficiency syndrome. Diagnostic features and outcome of treatment. Ann Intern Med 1986;104:234-40.
Saag MS, Cloud GA, Graybill JR, et al. A comparison of itraconazole versus fluconazole as maintenance therapy for AIDS-associated cryptococcal meningitis. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clin Infect Dis 1999;28:291-6.
Mussini C, Pezzotti P, Miro JM, et al. Discontinuation of maintenance therapy for cryptococcal meningitis in patients with AIDS treated with highly active antiretroviral therapy: an international observational study. Clin Infect Dis 2004;38:565-71.
Kirk O, Reiss P, Uberti-Foppa C, et al. Safe interruption of maintenance therapy against previous infection with four common HIV-associated opportunistic pathogens during potent antiretroviral therapy. Ann Intern Med 2002;137:239-50.
Lortholary O, Fontanet A, Memain N, et al. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS 2005;19:1043-9.
Shelburne SA 3rd, Darcourt J, White AC Jr, et al. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis 2005;40:1049-52.