Justin CY WU
The advent of highly active antiretroviral therapy (HAART) has substantially changed the pattern of gastrointestinal (GI) diseases in recent years.1 While the incidence of opportunistic infection and antibiotic related diarrhoeal conditions has declined with HAART and immune reconstitution in HIV patients, HAART related GI side effects, notably hepatotoxicity, are increasingly common nowadays. Furthermore, the prolonged life expectancy contributed by HAART leads to emergence of new long-term problems such as HBV and HCV co-infection, HAART related metabolic complications and hepatotoxicity.This chapter covers GI manifestations which are organised according to the anatomical site (oral, pancreas, hepatitis and cholangiopathy) or symptomatology (dysphagia, anorexia, nausea and vomiting, diarrhoea).
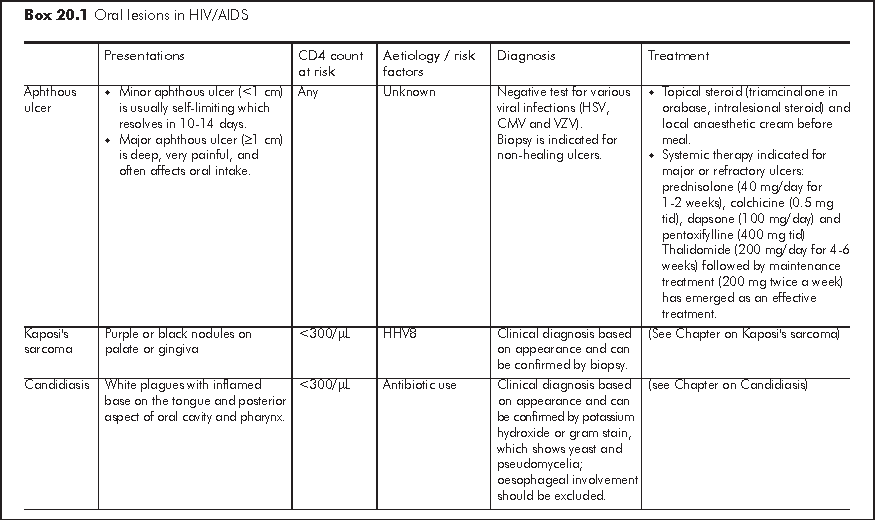
HIV infection is associated with various oral lesions, which results in pain and reduced oral intake. The three commonest oral lesions include aphthous ulcer, xerostomia and oral candidiasis.2 These oral lesions often have pathonomonic features and typical sites of involvement that allow clinical diagnosis. Some of the oral lesions may be part of manifestation of more extensive gastrointestinal or even systemic diseases especially in patients with CD4<300/μL. Therefore, a systemic survey of symptoms is necessary. Assessment of nutritional status is essential as oral intake is often impaired (Box 20.1).

Gingivitis and periodontis are caused by anaerobic bacteria. It can be divided into 4 phases: gingival erythema, necrotising gingivitis, necrotising periodontis (complicated by loose teeth), and necrotising stomatitis (complicated by removable teeth). The usual initial presentations are gum pain and bleeding. Treatment includes routine dental care and topical antiseptic such as chlorhexidine mouth wash. Metronidazole (400 mg tid), clindamycin (300 mg tid) or amoxicillin / clavulanate (1 g bid) for 1-2 weeks is useful in severe cases. Curettage and debridement may be required for necrotising complications.
Salivary gland enlargement and dry mouth may occur in HIV patients. CT scan is useful to distinguish between cystic and solid lesions. Fine needle aspiration permits microbiologic analysis and therapeutic decompression and biopsy may be required to exclude malignancy. Xerostomia can be treated with sugarless chewing gum and pilocarpine in refractory cases.
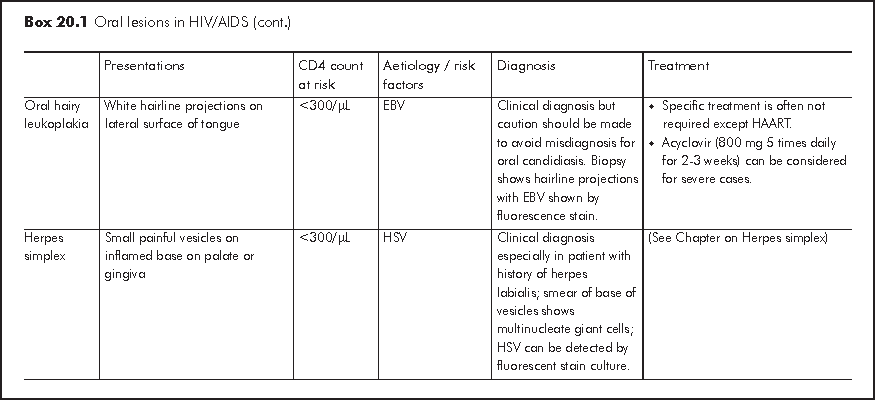
Oesophagitis should be considered in patients with dysphagia but xerostomia and poor dentition due to gum disease are often overlooked. Odynophagia usually suggests severe oesophageal mucosal lesions. Examination of oral cavity and nutritional assessment should be performed. Endoscopy is essential for evaluation of dysphagia and allows confirmation of diagnosis by biopsy. The main causes are candidiasis, CMV infection and herpes simplex (Box 20.2).
Anorexia often occurs in the setting of systemic illnesses such as neoplastic disease and infection. It is also related to local GI pathology that causes dysphagia, odynophagia and other upper gastrointestinal symptoms. Nausea and vomiting are often manifestations of systemic illnesses rather than gastrointestinal diseases in HIV patients. Medications, such as antiretroviral therapy and antibiotics, often cause GI upsets. Protease inhibitors, notably ritonavir (RTV), indinavir (IDV), boosted lopinavir (LPV/r) and boosted tipranavir (TPV/r), are commonly associated with dose-related GI side effects. Upper GI symptoms are also common presenting features of metabolic conditions such as adrenal insufficiency and lactic acidosis. Hypogonadism can lead to anorexia. Neuropsychiatric causes such as depression and intracranial pathology should also be ruled out if there are concomitant mood change and headache.
Review of medication history and systemic survey of associated symptoms are essential for evaluation. Lactic acid level, morning testosterone and low dose short synacthen test are useful for exclusion of endocrine disorders. Temporary withdrawal of HAART or change of regimen may be necessary to evaluate the GI side effect of individual ARTs. Water soluble contrast study or endoscopy may be required for patients with suspected gastric outlet obstruction.
Treatment of the underlying disease or readjustment of antiretroviral regimen often results in resolution of anorexia. Appetite stimulants can improve caloric intake and result in weight gain but no survival benefit or reduction in incidence of opportunistic infections has been documented. Megestrol (400-800 mg po daily) is a synthetic progestin that promotes weight pain in patients with HIV infection and neoplastic disease. However, weight gain is mostly fat and the increased calorie intake may not be sustained. It may lower testosterone levels, which leads to impotency and muscle wasting. Combined use of testosterone replacement and isometric resistance exercises are therefore recommended. Other major or common side effects include adrenal insufficiency, diabetes mellitus and diarrhoea. Dronabinol (2.5 mg bid) is the psychoactive ingredient of marijuana. CNS side effects (euphoria, confusion, anxiety), potential for abuse and drug interactions with psychotropic agents (especially tricyclic antidepressant) limit its application. It should only be reserved as second-line alternative to Megestrol. Prochlorperazine (5-10 mg q6-8h), metoclopramide (10 mg q6-8h), and dimenhydrinate 50 mg q6-8h are the first-line treatments for nausea and vomiting. Ondansetron (0.2 mg/kg) is an effective antiemetic for chemotherapy-related nausea and vomiting.
Nutritional supplementation by enteral alimentation is essential. Temporary or permanent percutaneous endoscopic gastrostomy can be considered for patients with severe dysphagia or anorexia. The use of long-term total parenteral nutrition should be reserved for patients in whom enteral nutrition is contraindicated and a meaningful life expectancy is anticipated.
Upper GI bleeding is relatively uncommon in HIV patients.3 Although peptic ulcer is the commonest cause of upper GI bleeding, the prevalence of Helicobacter pylori and peptic ulcer are somehow lower in HIV patients. The higher risk of progression to cirrhosis due to HIV and HBV or HCV co-infection may increase the risk of variceal bleeding with prolonged life expectancy of HIV patients.
Massive upper GI bleeding can be caused by diseases peculiar to HIV/AIDS or patients with severe immunodeficiency. Idiopathic oesophageal ulcer can be complicated by severe upper GI bleeding in HIV patients of any status. Kaposi's sarcoma and CMV infection are the two common causes of upper GI bleeding in HIV patients with CD4 <200/μL. Other rare causes include gastrointestinal bacillary angiomatosis, gastric non-Hodgkin's lymphoma and histoplasmosis.
In pre-HAART era, upper GI bleeding was associated with significant risk of recurrent bleeding and mortality in HIV patients. This was attributed to severe nature of the bleeding lesion (oesophageal ulcer, CMV ulcer, etc.), immunodeficiency, and HIV-related thrombocytopaenia. The introduction of HAART, notably protease inhibitor, has improved the outcome of upper GI bleeding. Endoscopic therapy remains the most important diagnostic investigation and it provides effective treatment for severe upper GI bleeding. With the advent of high dose IV infusion of proton pump inhibitor as an adjunct to endoscopic treatment of severe peptic ulcer bleeding, the issues of drug-drug interaction with antiretroviral drugs need to be recognised.
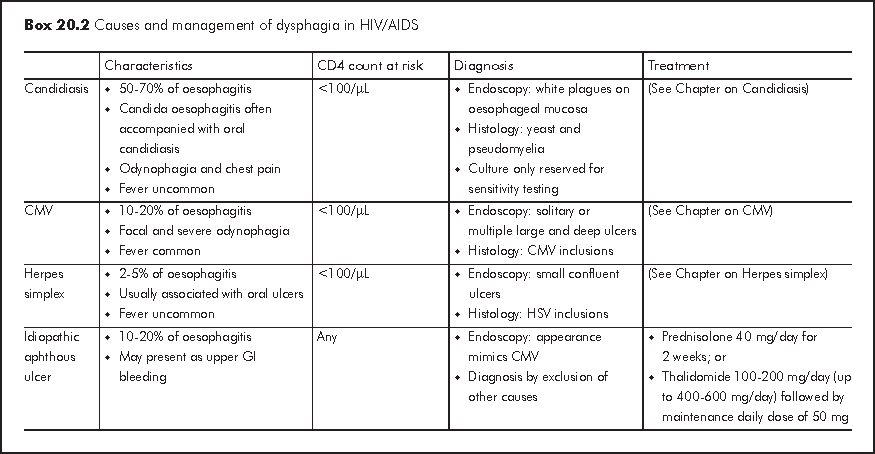
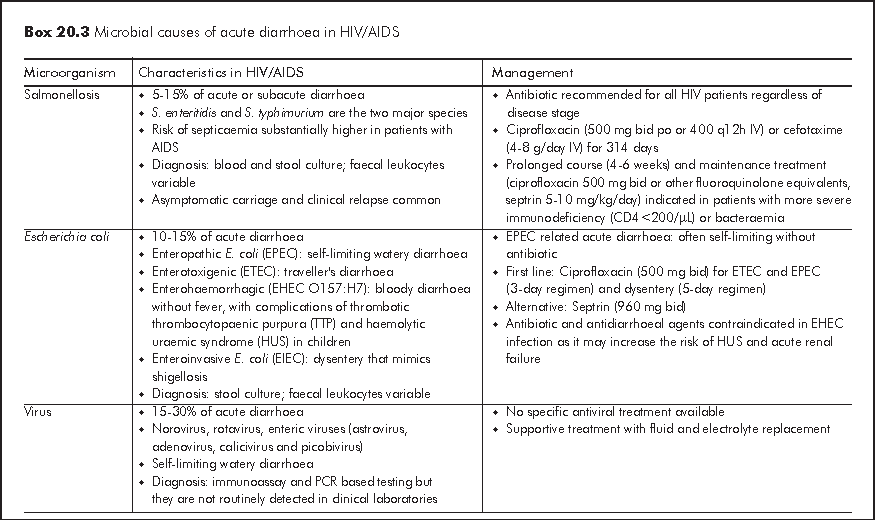
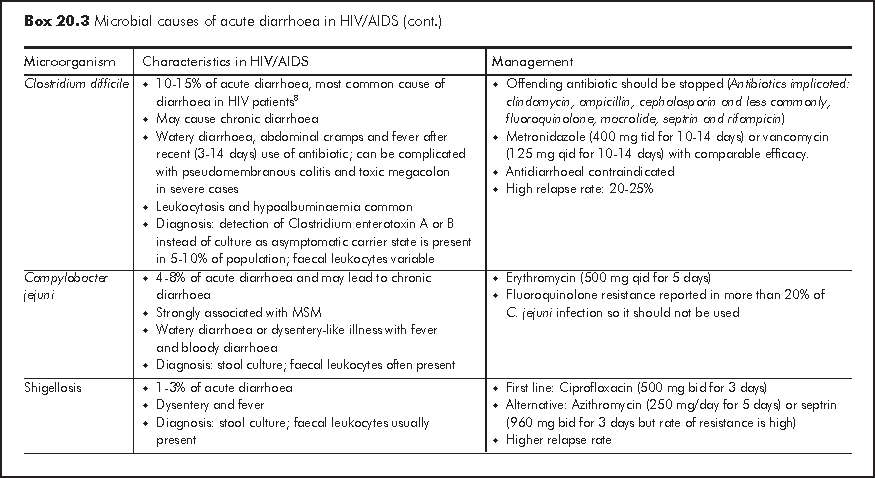
Acute diarrhoea is generally defined as >=3 loose or watery stools for 3-10 days. Bacterial and viral infections account for most of acute diarrhoeal illnesses in HIV patients with any CD4 count and these causative organisms are also responsible for infections in immunocompetent hosts (Box 20.3). Acute diarrhoea presents as either gastroenteritis with watery diarrhoea or dysentery, which is characterised by bloody diarrhoea and fever. Acute dysentery is classically caused by shigellosis, Campylobacter jejuni and enteroinvasive E. coli. Some causative organisms are strongly associated with men having sex with men (MSM) owing to person-to-person transmission through anal intercourse. Both ARTs (especially protease inhibitors: nelfinavir (NFV), LPV/r, saquinavir (SQV) and didanosine (ddI)) and antibiotics are common non-infective causes of acute diarrhoea. It is important to exclude HAART related lactic acidosis, which may presents as acute diarrhoea. Travel history, recent medication history and involvement of other close contacts often give important diagnostic clues. Faecal leukocytes are variable so it does not have high diagnostic value. Risk of severe complications such as septicaemia is much higher in HIV patients and therefore blood culture should be performed in patients with fever.
The indication of antibiotic treatment for acute infection and maintenance is also different from immunocompetent hosts because of higher risk of relapse, septicaemia and progression to chronic diarrhoea. Empirical antibiotic for bloody diarrhoea without fever is not recommended unless a positive bacterial cause other than Enterohaemorrhagic E. coli (EHEC O157:H7) is identified. Drug induced diarrhoea responds readily to antidiarrhoeal agent and dietary fiber. No cause can be identified in 25-40% of HIV patients with acute diarrhoea despite microbial investigations and exclusion of predisposing factors. This may partly be attributed to prior use of antibiotics before stool investigation. Empirical ciprofloxacin (500 mg bid for 5 days) with or without metronidazole has been advocated for treatment of severe idiopathic acute diarrhoea. A diagnostic algorithm is illustrated in Algorithm 20(A).
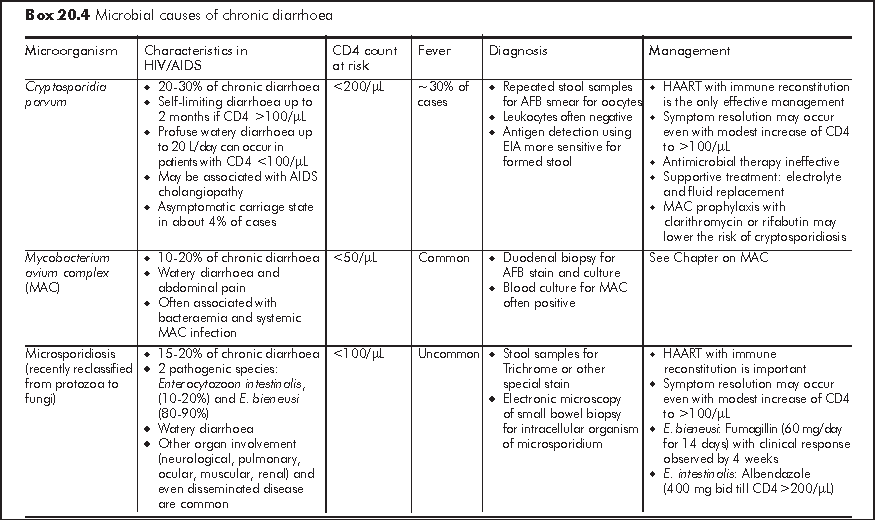
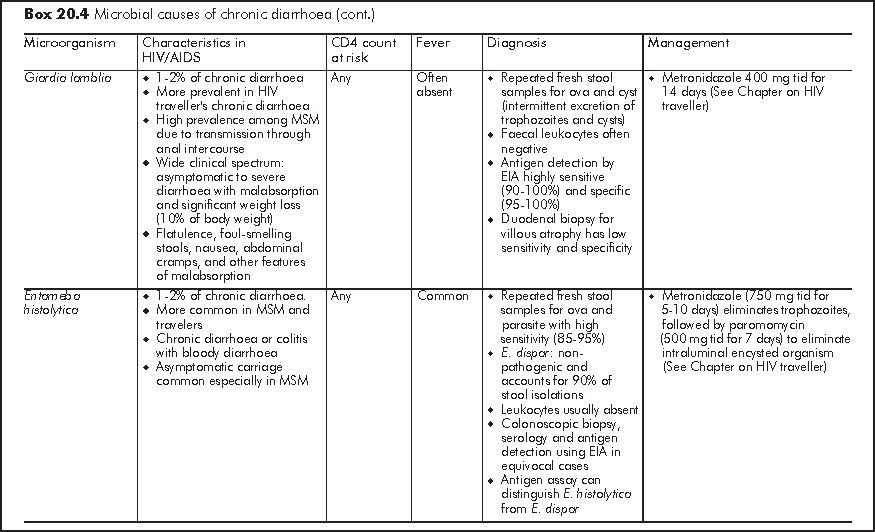
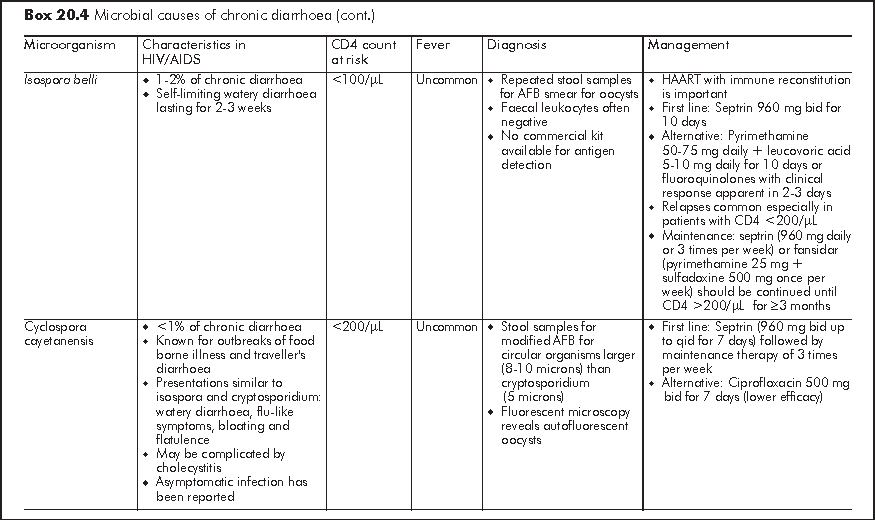
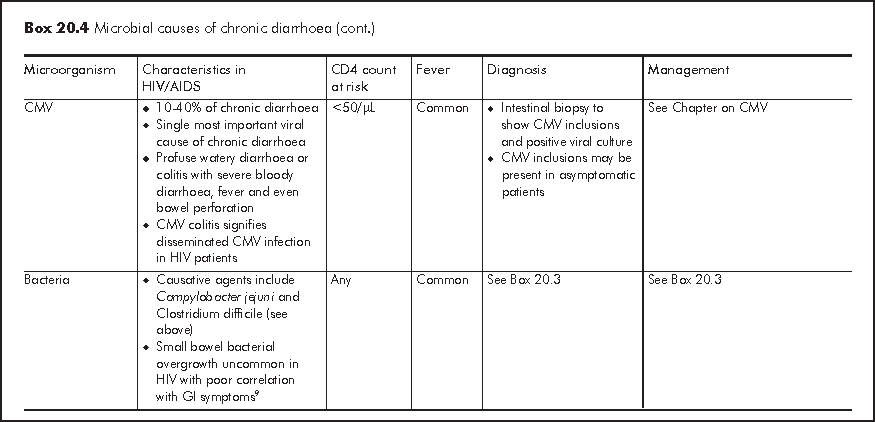
Chronic diarrhoea generally refers to persistent loose or watery stool of 2 times per day for >=30 days. It is often caused by opportunistic infections or parasitic infections which also affect immunocompetent host (Box 20.4). Similar to acute diarrhoea, some causative organisms are strongly associated with MSM owing to person-to-person transmission through anal intercourse. Symptomatology of diarrhoea, travel history and medication history give important clues to the underlying aetiology. Chronic diarrhoea due to small bowel diseases is often watery and large-volume in nature; this may be accompanied with bloating, steatorrhoea, malabsorption and profound weight loss. Chronic diarrhoea due to large bowel diseases is characterised by frequent small-volume diarrhoea and occasionally blood and mucus in stool. Patients with chronic diarrhoea should be assessed for stigmata of malabsorption and nutritional status.4 Liaison with microbiological laboratory should be made for stool microbial investigations for proper timing of sample collection and appropriate use of special stain (e.g. modified AFB, trichrome, etc). Faecal leukocytes are variable so it does not have high diagnostic value. Endoscopy has an important diagnostic role in over half of HIV/AIDS patients with chronic diarrhoea who have negative stool studies and no response to empiric treatment.5 Specific treatment guided by endoscopy leads to recovery in most patients. Upper endoscopy should be considered first in patients with small bowel diarrhoea or malabsorption, whereas lower endoscopy is more appropriate for bloody diarrhoea or other lower GI symptoms such as tenesmus. Flexible sigmoidoscopy is considered as cost-effective lower GI endoscopic investigation but colonoscopy should follow a negative sigmoidoscopy to avoid missing proximal colonic pathology.
Non-infective causes affect 2-10% of general population. For HIV/AIDS, these could range from neoplasm, medications to idiopathic in origin:
Neoplasm (See Chapters on Kaposi's sarcoma and lymphoma) - Kaposi's sarcoma and non-Hodgkin's lymphoma (NHL) may present with large volume diarrhoea or gastrointestinal bleeding. Forty percent of Kaposi's sarcoma has GI involvement, whereas NHL tends to present as late stage with prominent B symptoms. Endoscopy is essential but histological diagnosis by mucosal biopsy for Kaposi's sarcoma may yield false negative results because of its submucosal origin.
Medications - Chronic diarrhoea may occur in patients on protease inhibitor (see above) and medication-related diarrhoea should be considered if there is no associated or systemic symptom. It usually responds readily to antidiarrhoeal agent or dietary fiber.
Idiopathic - No cause is identified in 20-30% of chronic diarrhoea in HIV patients. This pathogen-negative chronic diarrhoea is also known as HIV enteropathy and is characterised by low volume watery diarrhoea. Small intestinal biopsy shows villous atrophy or decreased villous: crypt ratio with no increase in intraepithelial lymphocytes and other microbial evaluations are negative. Idiopathic chronic diarrhoea is readily controlled by antidiarrhoeal agent (Loperamide 4 mg tid).
Proctitis is common in HIV patients especially in MSM with receptive anal intercourse. Infectious diseases account for most differential diagnoses. About half of cases remain undiagnosed and multiple coincident infections, including concomitant infective colitis, are common. The four most common infectious causes include gonorrhoea, herpes simplex, chlamydia, and syphilis. In contrast to colitis due to other infectious disease, proctitis due to sexually transmitted infections is characterised by local symptoms including tenesmus and prominent purulent rectal discharge with paucity of systemic symptoms such as fever. Lymphogranuloma venereum (LGV) has also been implicated as a cause of proctitis in recent years. The presence of fever and inguinal lymphadenitis together with proctitis should raise the suspicion of LGV.
Owing to the high risk of transmission of both sexually transmitted illness and HIV infection and high prevalence of concomitant infections, HIV patients with proctitis should be broadly screened for various sexually transmitted diseases. Empirical therapy for gonorrhoea and chlamydia should be administered without delay while testing for specific agents is pending. A thorough contact investigation, treatment and prophylaxis should be initiated where appropriate (See Chapter on STI).
Lower GI bleeding is less common than upper GI bleeding patients, and majority of these cases are related to HIV related colonic diseases such as CMV colitis and idiopathic colonic ulcer.6 Haemorrhoidal bleeding accounts for only a small proportion. Other common causes observed in general population, which include colorectal cancer, diverticulosis and angiodysplasia, are rare in HIV patients because these conditions mainly affect elderly population. The incidence of lower GI bleeding due to these conditions may increase with the improved survival of HIV patients. Colonoscopy is essential for lower GI bleeding in HIV patients as this often signifies significant colonic pathology. Prognosis of lower GI bleeding remains poor because of severe immunodeficiency in these patients.
Condyloma acuminata is the commonest anorectal infection in MSM and HIV infection further increases the risk. It is caused by HPV infection and may progress to anal squamous intraepithelial lesion (SIL) and malignant transformation to squamous cell carcinoma. Although anal cancer is uncommon, there is an increase in incidence particularly in MSM.
In the pre-HAART era, liver dysfunction in HIV-infected patients is primarily related to opportunistic infections, neoplasm such as lymphoma and Kaposi's sarcoma, and drug-related hepatitis caused by antibiotics. These events reflected the severe immunodeficiency state of patients and were associated with very poor prognosis. The advent of HAART resulted in a significant decrease in morbidity and mortality among HIV-infected patients and modified the pattern of liver dysfunction in HIV infection. In contrast to the decline in incidence of gastrointestinal diseases, deranged liver function is increasingly common in HIV patients and is one of the leading causes of morbidity and mortality nowadays.
The differential diagnosis of deranged liver function in an HIV-infected patient on HAART includes drug-induced toxicity (isoniazid, PIs, NNRTIs, statins), acute hepatitis A infection, chronic hepatitis B (HBV) and C (HCV) coinfection, alcohol or substance abuse (cocaine and methamphetamine), nonalcoholic steatohepatitis (NASH), immune reconstitution syndrome,7 systemic opportunistic infections, and neoplasms. In HAART era, opportunistic infection such as MAC and CMV only accounts for minority of deranged liver function in HIV patients while HAART is responsible for a significant proportion of liver derangement. About 5-10% of HIV patients on HAART develop liver derangement. Immune reconstitution syndrome can lead to liver derangement after commencement of HAART. Liver derangement can also be a drug-specific adverse reaction. NVP (nevirapine) is well reported to cause hepatitis. d4T (stavudine) and, less commonly, AZT (zidovudine) and ddI (didarosine) are associated with lactic acidosis and steatosis. There are interactions between various mechanisms of hepatic damage. For example, HCV and HBV co-infection has been shown to increase the risk of hepatotoxicity caused by PI and NNRTI.
Review of medical history (e.g. use of statin for hyperlipidaemia, HAART, anti-TB treatment, diabetes mellitus, viral hepatitis status) and social history (substance abuse, alcohol) is essential for diagnostic work-up of patients with hepatitis. Ultrasound is the investigation of choice when biliary disease is suspected. Percutaneous liver biopsy should be reserved for patients in whom a treatable cause of parenchymal liver disease is suspected and when other noninvasive tests are not conclusive. Optimal management of deranged liver function is not well established and the treatment goal is to determine the aetiology and to minimise progressive liver damage.
AIDS cholangiopathy is a syndrome of biliary obstruction resulting from infection-associated strictures predominantly at intrahepatic ducts. It affects late stage AIDS patients with CD4 count <100/μL. Cryptosporium parvum is the most common pathogen but CMV, microsporidium, and cyclospora have also been implicated. No causative organism is identified in 20-40% of cases. AIDS cholangiopathy is classified according to the site of lesion into papillary stenosis, sclerosing cholangitis without papillary stenosis and isolated duct stricture. It typically presents with right upper quadrant pain and cholestatic pattern of liver derangement. Severe abdominal pain is more common in papillary stenosis. Diarrhoea is prevalent as part of cryptosporidiosis but fever and jaundice are less common.
Diagnosis is established by endoscopic retrograde cholangio-pancreatography (ERCP) but ultrasound is a more cost-effective initial investigation with high sensitivity (75-97%) and specificity (up to 100%). Despite its microbial origin, antimicrobial treatment is largely ineffective except for CMV infection. Endoscopic therapy is the mainstay of treatment. Sphincterotomy provides marked symptomatic relief in patients with papillary stenosis while endoscopic stenting is indicated for bile duct strictures. Sclerosing cholangitis without papillary stenosis is not amenable for endoscopic treatment but there are reported clinical improvement with the use of ursodeoxycholic acid (UDCA, 300 mg tid) in patients with intrahepatic ductal disease and marked liver derangement. Despite endoscopic and medical treatment, the prognosis of AIDS cholangiopathy remains poor with median survival of only 9 months. However, survival benefit has been reported with the use of HAART.
Acute pancreatitis occurs in about 5% of HIV patients, which is much more common than general population. It also tends to run a more complicated course in HIV patients with higher rate of prolonged hospitalisation and mortality. Apart from alcohol and gallstone, a number of other causes are peculiar to HIV patients. Acute pancreatitis can be part of primary HIV infection but more frequently related to medications. Antiretroviral drugs, notably ddI and d4T and less frequently 3TC, RTV, LPV/r, are associated with acute pancreatitis. The possible mechanisms of ART induced pancreatitis include lactic acidosis (nucleoside reverse transcriptase inhibitor) and hypertriglyceridaemia (protease inhibitor). It can also be related to adverse reactions resulting from the treatment of opportunistic infections, which include pentamidine, septrin, paromomycin, sulphonamide, erythromycin, rifampicin and isoniazid. Opportuisitic infections including CMV, Pneumocystis jiroveci, MAC, and cryptococcus, cryptosporidium and toxoplasmosis, are rare causes of acute pancreatitis in HAART era.
Diagnosis is confirmed by an elevated serum amylase level to 3 times the upper limit of normal. Serum lipase is as sensitive as amylase with higher specificity but it is not readily available. Ultrasound is relatively insensitive for diagnosis but it may detect dilated bile ducts in biliary pancreatitis. CT scan is an important imaging test for diagnosis, assessment of severity and detection of intra-abdominal complications such as pseudocyst. It should be considered in patients with poor response to initial conservative treatment, suspicion of intra-abdominal complications or pancreatic necrosis.
The treatment of acute pancreatitis in HIV is largely supportive (bowel rest, analgesia) apart from treating underlying cause (e.g. drug withdrawal, treatment of opportunistic infection). Early (within 24 hours) ERCP should be considered for patients with biliary pancreatitis. Conventional prognostic indicators such as Ranson and modified Glasgow scales have poor predictive value of severity in HIV patients. The APACHE II scale (Acute Physiology and Chronic Health Evaluation II), which contains markers of immunosuppression, is the only acceptable clinical indicator of severity (sensitivity 73%; specificity 68%; positive predictive value 70%; negative predictive value 71%). The presence of AIDS or leukopaenia also predict poor prognosis.
With the prolonged life expectancy of HIV patients in HAART, the impact and relationships between HIV infection and other gastrointestinal diseases in advanced age require further evaluation. In Hong Kong, the incidence of colorectal cancer has been increasing in a remarkable rate and anal neoplasia is associated with MSM with HIV infection. The policy of colorectal cancer screening in HIV patients, particularly those with age over 50, needs to be defined. The high prevalence of psychological morbidity in HIV patients may be associated with functional gastrointestinal disorder, such as functional dyspepsia and irritable bowel syndrome, which leads to increasing demand for investigations to exclude other HIV related complications. HIV patients are also prone to develop nonalcoholic steatohepatitis because of HAART related metabolic complications such as hyperlipidaemia and diabetes mellitus. The natural course of NASH and its long-term complication remain to be determined.
1. Wilcox CM, Rabeneck L, Friedman S. AGA technical review: malnutrition and cachexia, chronic diarrhea, and hepatobiliary disease in patients with human immunodeficiency virus infection. Gastroenterology 1996;111:1724-52.
2. Guerrant RL, Van Gilder T, Steiner TS, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis 2001;32:331-51.
3. Pol S, Lebray P, Vallet-Pichard A. HIV infection and hepatic enzyme abnormalities: intricacies of the pathogenic mechanisms. Clin Infect Dis 2004;38 Suppl 2:S65-72.
Monkemuller KE, Call SA, Lazenby AJ, Wilcox CM. Declining prevalence of opportunistic gastrointestinal disease in the era of combination antiretroviral therapy. Am J Gastroenterol 2000;95:457-62.
Tsang PC, Samaranayake LP. Oral manifestations of HIV infection in a group of predominantly ethnic Chinese. J Oral Pathol Med 1999;28:122-7.
Bini EJ, Micale PL, Weinshel EH. Risk factors for rebleeding and mortality from acute upper gastrointestinal hemorrhage in human immunodeficiency virus infection. Am J Gastroenterol 1999;94:358-63.
Call SA, Heudebert G, Saag M, Wilcox CM. The changing etiology of chronic diarrhea in HIV-infected patients with CD4 cell counts less than 200 cells/mm3. Am J Gastroenterol 2000;95:3142-6.
Wei SC, Hung CC, Chen MY, Wang CY, Chuang CY, Wong JM. Endoscopy in acquired immunodeficiency syndrome patients with diarrhea and negative stool studies. Gastrointest Endosc 2000;51(4 Pt 1):427-32.
Chalasani N, Wilcox CM. Etiology and outcome of lower gastrointestinal bleeding in patients with AIDS. Am J Gastroenterol 1998;93:175-8.
Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis 2006;42:418-27.
Sanchez TH, Brooks JT, Sullivan PS, et al. Bacterial diarrhea in persons with HIV infection, United States, 1992-2002. Clin Infect Dis 2005;41:1621-7.
Wilcox CM, Waites KB, Smith PD. No relationship between gastric pH, small bowel bacterial colonisation, and diarrhoea in HIV-1 infected patients. Gut 1999;44:101-5.