Patrick CK LI
Neurological complications affect a significant proportion of HIV-infected individuals and are important causes of death and morbidity. With progressive deterioration in immune function, the brain and nervous system become susceptible to a range of opportunistic infections (OIs) and tumours. The most commonly encountered OIs are cryptococcosis, tuberculosis, cytomegalovirus (CMV) disease, toxoplasmosis, progressive multifocal leucoencephalopathy (PML) and primary central nervous system lymphoma (PCNSL). In addition, HIV can directly affect the nervous system and cause HIV-associated dementia (HAD), distal sensory peripheral neuropathy (DSPN), myopathy and vacuolar myelopathy. Following the availability of highly active antiretroviral therapy (HAART) with resultant immune restoration, the incidence of the various OIs and tumours as well as HAD has significantly decreased. However, the patients may develop Immune Reconstitution Disease (IRD) with paradoxical deterioration of neurological symptoms and signs during the first few weeks after initiation of HAART. Side effects from antiretroviral agents and drugs for treatment of OIs may likewise affect the nervous system. In addition, long-term HAART may lead to metabolic disturbances which can increase the risk of cerebrovascular disease.
Diagnosis of neurological complications in HIV-infected individuals is often difficult. Clinical presentations may be subtle or atypical; dual infections frequently coexist, and a number of differential diagnoses may need to be considered. Laboratory and radiological investigations may only yield indirect information on the underlying aetiological agent. HIV itself can cause non-specific abnormalities in the cerebrospinal fluid (CSF) including lymphocytic pleocytosis and increased protein level even in the asymptomatic patients while advanced immunodeficiency can dampen the inflammatory response in the CSF. Access to lesion in the central nervous system for direct histopathological confirmation is often not feasible. Appropriate laboratory and radiological investigations may be applied on a rational basis or empirical therapy prescribed basing on the presumptive diagnosis.
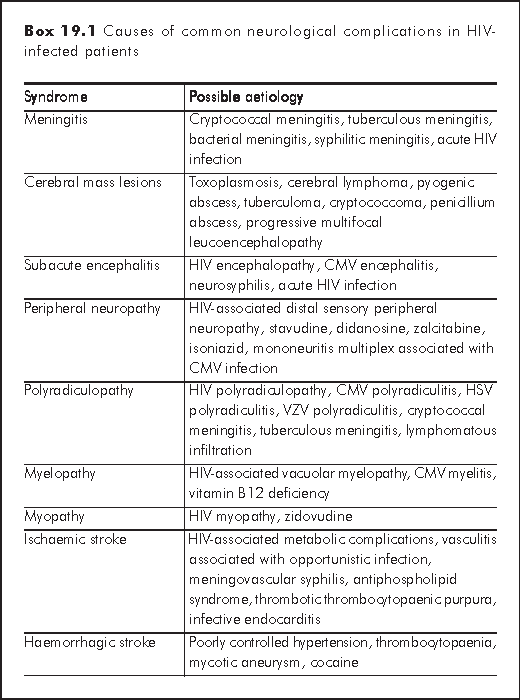
This chapter presents a syndrome based approach to the management of adult HIV-infected patients with neurological complications. Detailed information on individual OIs and tumours as well as paediatric neurological manifestations are described in other chapters of the manual. Common syndromes and their possible causes are listed in Box 19.1. An algorithm is recommended to illustrate the approach.

Clinical features of meningitic illness in the HIV-infected patient may include fever, headache, nausea and vomiting, neck pain, photophobia, drowsiness or cranial nerve symptoms. On physical examination, there may be neck rigidity, positive Kernig's sign, papilloedema, cranial nerve palsies or impaired conscious level. The clinical presentation of meningitis in the setting of HIV infection may be atypical and consists only of non-specific symptoms such as deterioration in general condition.
The commoner infectious agents causing meningitic illness in HIV-infected patients are Cryptococcus neoformans and Mycobacterium tuberculosis (MTB). Bacterial meningitis may also occur in association with HIV infection. In addition, acute HIV infection may cause aseptic meningitis associated with seroconversion. Occasionally, neurosyphilis may present with a meningitic illness.
The patient should be reviewed for prior or coexisting tuberculosis or systemic fungal infection. Skin lesions and lymphadenopathy are sought so that scraping or aspiration cytology could be performed respectively to identify the aetiological agent. Serum should be tested for cryptococcal antigen and blood should be taken for fungal and mycobacterial culture. Chest radiograph should be performed to look for any associated pulmonary lesions. In the absence of focal neurological signs or papilloedema, lumbar puncture should be performed as soon as possible. CSF should be sent for microscopy, biochemistry, Gram smear and bacterial culture, acid-fast stain and mycobacterial culture, negative stain and fungal culture, as well as cryptococcal antigen. Polymerase chain reaction (PCR) for MTB should be performed if the test is available. CSF should also be sent for cytology examination in case of the rare occurrence of lymphomatous infiltration causing the meningitic illness, and venereal disease reference laboratory (VDRL) and/or fluorescent treponemal antibody (FTA) test for the possibility of neurosyphilis.
Computed tomography (CT) scan usually shows negative findings in HIV-infected patients presenting with pure meningitic illness. There may be cerebral atrophy or basal meningeal contrast enhancement. Sometimes clinically silent tuberculoma or cryptococcoma may be detected. Communicating hydrocephalus may be detected and would require close monitoring and specific treatment.
Cryptococcal and tuberculous meningitis are common causes the treatment for which are given in the respective chapters. Cryptococcal meningitis patients are generally treated with a combination of intravenous amphotericin B and oral flucytosine for at least 2 weeks. After this period and when the clinical condition has stabilised, the patient may be switched to high-dose oral fluconazole until the CSF has become sterilised. For patients with severe infection and high load of cryptococcus in the CSF, amphotericin B therapy may need to be extended to 4-6 weeks. The patient should then receive secondary prophylaxis with fluconazole 200 mg daily until the CD4 count has increased above 200/μL following HAART.
Unlike pulmonary tuberculosis, tuberculous meningitis (TBM) tends to occur with more advanced immunodeficiency with CD4 count below 50/μL. The diagnosis of TBM is less straight forward unless the patient is known to have coexisting tuberculosis, usually affecting the lungs. CSF examination for acid-fast bacilli (AFB) generally has a low yield and mycobacterial culture may take a few weeks to give a positive result. PCR for MTB may be helpful if a positive result is obtained but negative PCR does not completely exclude the diagnosis.
It may be necessary to administer empirical anti-tuberculous therapy in the clinical setting of meningitic illness in which bacterial cause is considered unlikely and CSF examination for yeasts and cryptococcal antigen yields negative result. The duration of treatment may have to be extended for patients who cannot receive isoniazid or rifampicin because of side effect. Corticosteroid therapy has been shown to reduce the mortality but not the disability from TBM. Initiation of HAART should normally be deferred until the patient had been stabilised on anti-tuberculous drugs and the clinician should recognise the possibility of paradoxical deterioration of meningitis from IRD shortly after initiation of HAART.
An important complication of cryptococcal or tuberculous meningitis is raised intracranial pressure with or without communicating hydrocephalus. This can result in the impairment of conscious level, deterioration in mobility as well as potentially irreversible visual loss. While the patient is receiving specific therapy, lumbar puncture should be performed periodically to monitor the CSF response and the CSF pressure should be recorded. Patients with high CSF pressure should undergo repeated lumbar puncture at short intervals so as to control the raised intracranial pressure. CT brain scan should also be repeated to detect any increase in the severity of hydrocephalus. Ventricular or lumbar drainage may be necessary for patients with clinical deterioration associated with persistent raised intracranial pressure or progressive hydrocephalus despite repeated lumbar puncture and appropriate anti-tuberculous therapy.
An HIV-infected patient with mass lesions in the brain may present with headache, focal motor weakness or sensory impairment, visual or speech disturbance, seizures and impaired conscious level. Physical examination may reveal hemiparesis, hemisensory impairment, dysphasia or visual field defect.
The commoner opportunistic diseases that may present with mass lesions in the brain include cryptococcoma, tuberculoma, toxoplasmosis, lymphoma and PML.
The patient should be assessed for prior or coexisting tuberculosis, systemic fungal infection as well as non-Hodgkin lymphoma (NHL). History of injection drug use may suggest the possibility of bacterial brain abscess. Physical examination should be performed to detect any skin lesion, lymphadenopathy, hepatosplenomegaly or cardiac murmur. Blood should be sent for culture and tested for toxoplasma antibody and fungal antigen. Chest radiograph should be performed to look for any pulmonary lesion. The aim is to identify any evidence of systemic disease process which might provide clues to the aetiology of the cerebral lesion.
CT scan with contrast should be performed to determine the number and location of the focal brain lesions, and such features as contrast ring enhancement, surrounding oedema, mass effect and midline shift. If the lesion could not be clearly identified on CT scan, as with lesions located in the posterior fossa or with little or no contrast enhancement, magnetic resonance imaging (MRI) should be performed for better delineation.
The management strategy varies with the radiological characteristics of the brain lesions on CT scan and MRI. Focal brain lesions with contrast ring enhancement, mass effect and surrounding oedema may be due to pyogenic brain abscess, tuberculoma, cerebral toxoplasmosis or lymphoma. If the patient has history of intravenous drug use or is clinically toxic with peripheral leucocytosis, high-dose antibiotics (ceftriaxone, metronidazole with or without cloxacillin) should be started as soon as possible after blood has been taken for bacterial culture. The lesions are more likely to represent tuberculoma or tuberculous abscess if the patient has active tuberculosis and negative toxoplasma serology, especially if meningeal enhancement is present on contrast CT scan. Anti-tuberculous regimen should be optimised and corticosteroid therapy may be added, especially if there is significant mass effect from the brain lesion or if the recently developed neurological symptoms could represent IRD following initiation of HAART.
In other clinical settings, the commonest cause of contrast-enhancing brain lesions is cerebral toxoplasmosis, especially if these are multiple and located in the basal ganglia or grey-white matter junction. Toxoplasmosis usually occurs in HIV-infected patients with CD4 count below 50/μL. The majority of patients are positive for anti-toxoplasma IgG antibody. Even if toxoplasma serology is negative, the patient should still receive empirical anti-toxoplasma therapy with pyrimethamine (200 mg for one dose, then 50-75 mg daily) and clindamycin (600 mg every 6 hours). Most patients with cerebral toxoplasmosis respond to therapy within 2 weeks. In the setting of negative toxoplasma serology, use of corticosteroid should be avoided unless the patient has significant mass effect causing severe clinical symptoms, so as not to give a misleading impression of favourable response to therapy.
PCNSL is the other important differential diagnosis of contrast-enhancing cerebral mass lesion. It may have similar radiological appearances to cerebral toxoplasmosis and diagnosis may depend on stereotactic biopsy of the lesion following failure to respond to a two-week empirical course of anti-toxoplasma therapy. Alternatively, thallium-201 single-photon emission computerised tomography (SPECT) and PCR for EBV DNA in the CSF may help to differentiate neoplastic from infective causes of cerebral mass lesion. Approach to management is detailed in Chapter 33.
Lesions predominantly affecting the white matter with no mass effect or contrast enhancement are usually due to PML (refer to chapter 30). Diagnosis is based on the compatible clinical and MRI features. The frontal and parieto-occipital lobes are most commonly affected with large single or multiple lesions with scalloping at the grey-white junction. PCR examination of the CSF for JC virus (JCV) has a sensitivity of 75% and specificity of near to 100%. Brain biopsy may be necessary for patients with negative PCR for JCV on at least two examinations. There is no specific treatment for PML and the mainstay is to improve the immune function with HAART.
Some HIV-infected patients may develop more diffuse disease process affecting the brain function. The onset may be subacute or more insidious and the symptoms may range from impairment in cognitive function, change in personality or mood, deterioration in motor function to confusion or clouding of consciousness. Such clinical presentation is usually due to HIV encephalopathy, resulting in the syndrome known as HAD. Differential diagnoses include CMV encephalitis and neurosyphilis. Acute HIV infection may also present with an encephalitic illness associated with seroconversion.
The patient should undergo investigations to identify any reversible causes of cognitive impairment or confusion. Fundoscopic examination should be performed to look for evidence of CMV retinitis. Blood should be tested for electrolytes, vitamin B12, folate, thyroid hormone level, cryptococcal antigen and VDRL. The possibility of depression should be considered and the patient should be referred to neuropsychologist to establish the baseline cognitive function and to monitor the progress following treatment. CSF should be examined for infective agents and electroencephalogram should be performed to look for any seizure activities. CT scan and MRI should also be arranged to identify any cerebral mass lesions.
A patient with HIV encephalopathy usually presents with the triad of cognitive, motor and behavioural disturbances. It can occur at any time during the HIV disease course but is more common with advanced immunodeficiency. There is insidious onset of intellectual decline, with forgetfulness, decreased concentration, slowing of thought process and deterioration in work performance. In its mildest form, the patients may have only minor cognitive motor disorder (MCMD) which may go unrecognised by the healthcare workers. Motor disturbances may manifest as clumsiness, limb weakness and unsteadiness of gait. The patient may have change in personality and become apathetic and withdrawn from the usual hobbies and social activities. This is followed by progressive decline in memory, language and other cognitive function ultimately resulting in severe psychomotor retardation, akinetic mutism, frank dementia and organic psychosis.
Physical examination is usually normal but may reveal hyperreflexia and frontal release signs, such as glabellar and snout reflexes. Neuropsychological tests show features suggestive of a subcortical dementia, with abnormalities mainly in psychomotor speed, verbal and non-verbal learning. The AIDS Clinical Trial Group (ACTG) has used timed gait, finger tapping (dominant hand), grooved pegboard and digit symbol as the screening tool for HAD. There is non-specific mild lymphocytic pleocytosis and increased protein level in the CSF. CT brain scan usually shows diffuse cerebral atrophy with ventricular dilatation and MRI may show T2-hyperintense signals mainly in the periventricular white matter and centrum semiovale with no mass effect or contrast enhancement. The diagnosis is usually established by exclusion of other infective causes of encephalitis.
Patient with a presumptive diagnosis of HAD should be initiated on HAART to control HIV replication. While some of the antiretroviral agents including protease inhibitors have only limited CNS penetration, no single combination has been shown to be superior to others in terms of effectiveness in treating HAD. If possible, zidovudine should be included in the regimen since the drug was shown to be effective in one clinical trial. For patients already receiving HAART, the CSF HIV RNA level may be checked to see if viral replication is adequately suppressed and the HAART regimen needs to be intensified. Alternative diagnoses should be considered in patients developing cognitive impairment while responding to HAART.
CMV infection and disease in the brain may be more common than is clinically recognised. It may take the form of diffuse micronodular encephalitis or ventriculoencephalitis. It generally occurs in the setting of advanced HIV disease with CD4 lymphocyte count below 50/μL. The onset with ventriculoencephalitis is more acute with rapidly progressive lethargy and confusion often associated with nystagmus and cranial nerve palsies. CMV encephalitis may run a indolent course with features mimicking a more aggressive form of HAD with greater propensity for delirium, confusion and focal neurological deficits. The patients generally run a rapidly progressive course leading to death within a few weeks. It may occur in the setting of CMV disease elsewhere in the body, and the patient may already be receiving ganciclovir therapy, raising the possibility of drug-resistant virus. Electrolyte disturbances such as hyponatraemia and hyperkalaemia may suggest presence of adrenal insufficiency from CMV adrenalitis.
On investigation, CT scan and MRI may show similar findings to HIV encephalitis with cerebral atrophy with ventricular dilatation and diffuse T2-hyperintense lesions in the white matter but there may be evidence of ventricular or meningeal enhancement. CSF may show polymorphonuclear pleocytosis as well as increased protein and decreased glucose level. PCR for CMV DNA is usually positive and is predictive of CMV encephalitis in the appropriate clinical context. Quantitative assay for CMV DNA in the CSF may have prognostic implications and can be used to monitor the response to treatment.
Patients with CMV encephalitis may not respond well to ganciclovir or foscarnet, especially if they are already on this treatment for systemic CMV disease. Combination therapy with ganciclovir (5 mg/kg every 12 hours) and foscarnet (60 mg/kg every 8 hours) may be administered in view of the disease severity and poor prognosis. Maintenance therapy with ganciclovir (5 mg/kg daily) and foscarnet (90 mg/kg daily) is necessary for patients with clinical response.
HIV-infected patients may develop complications due to disease process affecting the peripheral nerves, spinal nerve roots, spinal cord or muscles. The symptomatology is determined by the pattern of anatomical involvement and each is associated with different underlying aetiology. An acute inflammatory polyradiculoneuropathy (Guillain Barré syndrome) may occur early in the course of HIV infection, including the setting of acute HIV seroconversion illness. Mononeuritis multiplex affecting the cranial or peripheral nerves may also occur during early disease stage and may be due directly to HIV or coexisting CMV infection. More commonly the patients develop peripheral neuropathy which is predominantly sensory and affects the lower limbs. This is usually due to HIV-associated DSPN but may also be caused by nucleoside reverse transcriptase inhibitors such as stavudine, didanosine, zalcitabine or other potentially neurotoxic drugs like isoniazid, especially if more than one such agents are used in combination.
Less commonly the patient may present with progressive HIV polyradiculopathy during advanced stage of the disease and the main differential diagnoses are polyradiculitis associated with CMV, herpes simplex virus (HSV) or variciella zoster virus (VZV). Meningeal inflammation due to tuberculosis, neurosyphilis, cryptococcosis and lymphoma may also lead to a pattern of multiple spinal nerve roots involvement. Spinal cord involvement may be due to myelitis associated with acute HIV seroconversion illness or CMV disease. More insidious spinal cord disease with predominant involvement of the posterior column may be due to tabes dorsalis, subacute combined degeneration of the cord or HIV-associated vacuolar myelopathy. A proximal myopathy with ragged red fibres on muscle biopsy is associated with mitochondrial pathology from use of zidovudine.
The drug history should be reviewed and systematic workup should be conducted for other common causes of neuromuscular disorder. Blood tests should be performed for fasting glucose, creatine phosphokinase, vitamin B12 and folate levels, or any evidence of lactic acidosis associated with the nucleoside reverse transcriptase inhibitors. Patients developing neuropathy or polyradiculopathy should undergo nerve conduction studies and electromyography (EMG) to define the characteristics, extent and severity of the neuropathy. Nerve biopsy should be considered if there is presence of mixed sensorimotor involvement. If the clinical features are predominantly confined to the lower limbs, MRI should be performed to define any pathological changes in the spinal cord or cauda equina. If polyradiculitis is suspected, CSF should be examined for MTB, cryptococcal antigen, VDRL, HSV, VZV, CMV and abnormal cells suggesting presence of lymphoma.
HIV-associated DSPN usually occurs in the setting of advanced immunodeficiency. There is gradual onset of numbness, paraesthesia and burning discomfort in a glove-and-stocking distribution mainly affecting the lower limbs while muscle power is generally preserved. CMV polyradiculopathy tends to present with rapidly progressive pain, sensory loss, ascending flaccid paralysis and areflexia involving the lower limbs with bladder and bowel sphincter disturbance. This also occurs in the setting of advanced immunodeficiency with CD4 lymphocyte count below 50/μL. Nerve conduction studies usually show delayed or absent F waves while EMG reveals denervation in the lower limb muscles. CSF shows polymorphonuclear leucocytosis, raised protein and decreased glucose level and positivity for CMV DNA by PCR. MRI may show thickening and clumping of the affected nerve roots, enlargement of the conus medullaris and contrast enhancement of the leptomeninges. HIV-associated vacuolar myelopathy has a more insidious onset with slow progressive painless weakness, spasticity and sensory loss involving the lower limbs together with sphincter disturbance. There is usually no sensory level and if present should suggest an alternative diagnosis. It usually occurs in the setting of advanced immunodeficiency often in conjunction with HAD and DSPN. CSF examination is usually normal and MRI may show cord atrophy or some T2-hyperintensity in the posterior column.
The management of neuromuscular syndrome is mainly symptomatic. The offending drug should be identified and withdrawn. Immune function should be optimised with HAART. Specific therapy should be administered if CMV polyradiculitis is diagnosed. The neurological deficit may not improve despite treatment of the underlying aetiology especially in patients with severe myelopathy and radiculopathy. Physiotherapy and occupational therapy should be offered to help the patients overcome the physical disability. Painful sensory symptoms may require palliation with analgesics, tricyclic antidepressant and antiepileptic drugs. The latter includes carbamazepine, gabapentin, sodium valproate and lamotrigine. It should be noted that carbamazepine may result in drug interaction with the antiretroviral agents and its use should be avoided.
HIV-infected patients may also develop a stroke-like syndrome consisting of sudden onset of focal neurological dysfunction such as weakness, sensory loss, visual or speech disturbance. As the patients are surviving longer with HAART and many develop hypertension and metabolic complications including diabetes mellitus, hypercholesterolaemia and hypertriglyceridaemia, they become at risk of cerebrovascular disease. This may take the form of ischaemic stroke and intracranial haemorrhage.
They may also develop focal ischaemic brain injury from vasculitis or perivascular inflammation related to infectious such as tuberculosis, cryptococcosis and meningovascular syphilis. Ischaemic stroke may also develop in association with coagulopathy including anti-phospholipid syndrome and thrombotic thrombocytopaenic purpura as well as vasculopathy following radiotherapy for PCNSL. Intracranial haemorrhage may occur in relation to thrombocytopaenia or poorly controlled hypertension. Patients with history of injecting drug use may develop infective endocarditis which can lead to embolic stroke or haemorrhage from mycotic aneurysm. Cocaine use may also result in abrupt increase in blood pressure causing haemorrhage or vasospasm causing ischaemic stroke.
The clinical history should be reviewed for history of injecting or recreational drug use, hypertension, diabetes mellitus, hyperlipidaemia and smoking habit. Blood should be examined for platelet count, renal function tests, fasting glucose, cholesterol, triglyceride and VDRL. Blood culture, electrocardiogram and echocardiogram should be performed if cardiac source of embolus is suspected. Younger patients should also be tested for cardiolipin antibody and lupus anticoagulant. CT brain scan should be performed to differentiate ischaemic from haemorrhagic stroke.
Management should follow the same principles as for the usual patients with stroke. The should be admitted to organised stroke care programme with stabilisation of the vital signs, prevention of complications such as aspiration or deep vein thrombosis, and early rehabilitation. Underlying predisposing causes should be appropriately managed to prevent stroke recurrence and aspirin should be prescribed as secondary prophylaxis in patients with ischaemic stroke.
1. Evaluation and management of intracranial mass lesions in AIDS. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 1998;50:21-6.
2. Brew BJ. HIV neurology. Contemporary Neurology Series. Oxford: Oxford University Press, 2001.
3. McArthur JC, Haughey N, Gartner S, et al. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol 2003;9:205-21.
4. Verma S, Micsa E, Estanislao L, Simpson D. Neuromuscular complications in HIV. Curr Neurol Neurosci Rep 2004;4:62-7.
5. Bradley WG, Daroff RB, Fenichel GM. Neurological manifestations of human immunodeficiency virus infection in adults. Neurol Clin Pract 2004; 2:1581-1602.
6. Manji H, Miller R. The neurology of HIV infection. J Neurol Neurosurg Psychiatry 2004;75 Suppl 1:i29-35.
7. McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol 2005;4:543-55.
8. Nath A, Sacktor N. Influence of highly active antiretroviral therapy on persistence of HIV in the central nervous system. Curr Opin Neurol 2006;19:358-61.