Man-Po LEE
Lung is a major target organ for HIV infection that has been shown to be present in T and B lymphocytes, pulmonary fibroblasts, macrophages, Natural Killer cells, eosinophils, monocytes and dendritic cells. As a consequence, progressive quantitative and functional depression within the CD4 lymphocytes and other immunological subsets occur and render the patient more prone to a wide array of infectious and non-infectious complications.
In a prospective multi-centre trial investigating respiratory complications from 1988 to 1994, respiratory infections were more common at all CD4 cell strata than for HIV-uninfected controls. Over 98% of respiratory complications were infectious and the most frequent complications were acute bronchitis, bacterial pneumonia and PCP.1 The mortality and morbidity of HIV-infected patients have dramatically improved as a result of the introduction of highly active antiretroviral therapy (HAART). Besides, the spectrum of pulmonary diseases has changed significantly. An analysis of the Centers for Disease Control and Prevention's HIV Outpatient Study (HOPS) reported reductions in overall pulmonary mortality and morbidity between 1994 and 2003. HOPS study also demonstrated that pulmonary disease has declined as a cause of hospitalisation for HIV-infected patients compared with other causes. Prevalence, hospitalisations and mortality related to PCP have decreased too. However, some non-infectious pulmonary complications have not decreased as significantly since the widespread use of HAART.2
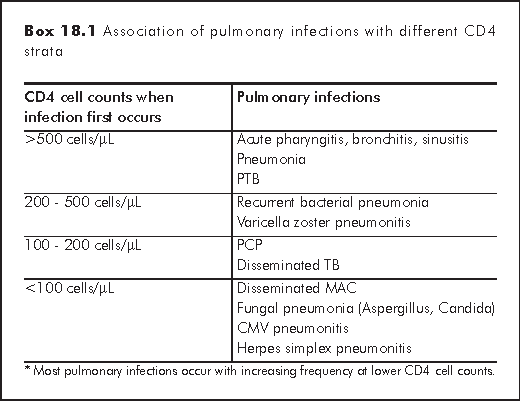
There are clear associations between immunosuppression, as indicated by the CD4 cell count, and the risk of developing specific pulmonary infections (Box 18.1).3 The most frequent respiratory diagnoses in the HIV-infected patients are upper respiratory tract infection, acute bronchitis, and acute sinusitis. They occur at all strata of CD4 cell counts and have higher rates compared with HIV-negative control. Recurrent bacterial pneumonia and pulmonary tuberculosis (PTB) occur more frequently in patients with CD4 cell counts less than 400 cells/μL; Pneumocystis pneumonia (PCP) and disseminated TB usually diagnose when CD4 cell counts drop below 200 cells/μL. Disseminated MAC, fungal pneumonia, and cytomegalovirus pneumonitis occur in patients with the most severe immunosuppression (CD4 cell counts less than 100 cells/μL).

Bacterial pneumonia occurs more frequently in HIV-infected patients than in the general population. Pneumonia occurs at any CD4 cell count but is especially common as HIV infection progresses. Rate of pneumonia is higher in intravenous drug users than other transmission categories. The spectrum of bacterial pathogens is similar to that of community-acquired pneumonia in the general population and Streptococcus pneumoniae remains the most common pathogen. Staphylococcus aureus and gram-negative organisms in particular Pseudomonas aerugionsa are seen more frequently in advanced disease. The incidence of bacteraemia is increased too. Treatment is similar to that for HIV-negative patients. TMP-SMZ (co-trimoxazole) for PCP prophylaxis may be effective in preventing bacterial pneumonia. Influenza and pneumococcal vaccination is indicated in HIV-infected patients.
Some unusual organisms can cause pneumonia in HIV-infected patients. Nocardia occurs in patients with defective cell-mediated immunity. It is a weakly acid-fast, Gram-positive, branching higher bacterium. The clinical manifestations are nonspecific, and may result in abscess formation. Rhodococcus species are also identified increasingly. The bacterium is facultative intracellular pathogen, causing granuloma formation with or without caseating necrosis. Both illnesses can mimic PTB.
PCP, MTB, MAC, fungal infections and CMV diseases are discussed in other chapters.
Pulmonary involvement is present in up to one third of patients with known Kaposi's sarcoma (KS). It usually follows the appearance of cutaneous disease. Intrathoracic involvement by KS may include parenchymal disease, endobronchial lesions, pleural disease, and adenopathy. The prognosis of pulmonary KS is poor with median survival of 2 to 10 months. However, there is significant reduction in mortality after the introduction of HAART and newer combination chemotherapy.
The incidence of intrathoracic manifestations of AIDS-associated lymphoma ranges from 6% to 31%. Lung involvement is usually seen in association with other sites of disease but occasionally it can be the initial or predominant site of disease. The median CD4 cell count has been noted to be lower in patients with pulmonary involvement than in those without. Chest radiographs may show effusions, multi-nodular infiltrates, consolidation, mass lesions, focal or diffuse interstitial infiltrates, and hilar adenopathy.
Epidemiological studies have suggested that lung cancer occurs more frequently in HIV-infected patients, but is often linked to the increased smoking rates. There is no definite association between the incidence and CD4 cell count. Adenocarcinoma has been the most common histology. Survival is significantly shorter for HIV-infected patients compared with HIV-negative subjects and outcomes of these patients remain poor despite HAART.4
Lymphocytic interstitial pneumonitis (LIP) is characterised histologically by diffuse infiltration with predominantly small lymphocytes and plasma cells in the alveolar septae and along lymphatic vessels. The exact cause is still not clearly defined. LIP is common in the paediatric HIV population, occurring in 22-75% of children with pulmonary disease. In contrast, it remains uncommon among adult HIV patients, accounting for 3% of adult HIV-related pulmonary pathology. Patients usually present with insidious onset of dyspnoea and chronic cough together with generalised lymphadenopathy, parotid swelling and hepatosplenomegaly. Chest radiograph may show bilateral reticulo-nodular shadowing.
The incidence of pulmonary hypertension in HIV-infected patients is estimated to be 0.5%, compared with 0.02% in the general population. Pulmonary hypertension occurs at all CD4 cell counts. The pathological findings are similar to primary pulmonary hypertension and characterised by plexogenic pulmonary arteriopathy together with thrombotic pulmonary arteriopathy and pulmonary venoocclusive disease. Initiation of HAART has been shown to decrease right ventricular systolic pressure and improve survival.
HIV-infected patients who have pulmonary diseases usually have respiratory symptoms such as dyspnoea, cough, sputum production, or wheezing. History frequently provides clues to the diagnosis of pulmonary diseases. Mode of HIV transmission may influence disease patterns: Men having sex with men (MSM), for example, are 10 times more likely to develop KS and intravenous drug users are prone to develop PTB and bacterial pneumonia. As mentioned earlier, the degree of immunosuppression has a significant influence on the spectrum of pulmonary diseases. Knowing patient's CD4 cell count can help to formulate the differential diagnoses. Checking use of prophylaxis is also important to estimate the risk of opportunistic infections.
Physical examination may reveal KS lesions or characteristic fungal skin lesions. The presence of peripheral lymphadenopathy may suggest TB, lymphoma, KS or lung cancer. Diagnosis can be made by needle aspiration or excisional biopsy of these lymph nodes.
Chest radiograph is a useful screening test for HIV-infected patients who complained of chest symptoms or unexplained constitutional symptoms, such as fever, night sweats, or weight loss. Pulmonary diseases in HIV-infected patients have different radiographic patterns. Patients with PCP typically present with bilateral pulmonary infiltrates in a perihilar distribution. Disseminated TB, disseminated fungal infections, CMV pneumonitis, KS and LIP also give rise to diffuse pulmonary infiltrates. On the other hand, bacterial pneumonia and PTB often results in focal lung infiltrates. Presence of hilar or mediastinal lymphadenopathy may suggest PTB, lymphoma, KS or lung cancer. Pleural effusion is rare in PCP but may be present in bacterial pneumonia, PTB, lymphoma and KS. It is important to note that normal chest radiographs have been described in patients with PCP (up to 26%) and PTB (up to 14%). Pulmonary diseases may sometimes have atypical radiographic patterns. PCP may occasionally present with solitary or multiple nodules, or upper-lobe infiltrates in patients receiving aerosolised pentamidine. Post-primary pattern of PTB, such as lower lobe infiltrates, with or without intrathoracic adenopathy and pleural effusions, is more common in HIV-infected patients than HIV-negative patients.
Computed tomography of thorax can sometimes provide additional information for the diagnosis of pulmonary diseases. High-resolution computed tomography is more sensitive than chest radiography in picking up PCP and may reveal extensive ground-glass attenuation or cystic lesions. CT scan may be useful in the diagnosis of PTB, especially in patients with intrathoracic adenopathy. KS typically produces a pattern of perivascular and perbronchial confluent opacities.
Pulse oximetry and arterial blood gas analysis measure the severity of gas exchange impairment of pulmonary diseases. They can be used as screening tools for the detection of PCP too. Patients with PCP may have normal chest radiograph and oxygen saturation at rest. Many studies have shown that pulse oximetry can be measured during exercise to screen for the presence of PCP with sensitivities ranging between 74% and 100%. However, patients without PCP may give positive results. One study showed that the specificity of exercise test was 77%.
Expectorated sputum examination and culture are initial microbiological tests for bacterial pneumonia and PTB. Staining expectorated sputum for acid-fast bacilli (AFB) has been reported to have a sensitivity of 31% to 89%. Conventional AFB culture can take 6-10 weeks but radiometric methods, such as the Bactec system, can shorten to 2-3 weeks.
The diagnostic yield of PCP is much improved by introducing sputum induction. Patients inhaled aerosol of 3% to 5% hypertonic saline generated by ultrasonic nebulizer. Induced sputum is then sent for staining with Wright-Giemsa, Papanicolaou, or methenamine silver stain. Studies showed that sputum induction has a diagnostic yield of 50% to 90%. Complications of sputum induction include cough and wheezy attack.
Bronchoscopy remains the most useful diagnostic method if screening tests fail to confirm the diagnosis of pulmonary diseases. Regarding PCP, the diagnostic yield from bronchoalveolar lavage (BAL) was considered sufficient to be used as the sole bronchoscopic modality for PCP diagnosis. However, numerous studies have reported a decreased yield from BAL following aerosolised pentamidine prophylaxis. The sensitivity of BAL was 62% for the aerosolised pentamidine group compared with 100% for the control group. Investigators have advocated several strategies to enhance the diagnostic yield in this situation such as multiple-lobe, site-directed BAL (defined as lavage at the most abnormal lobe on the chest radiograph), addition of transbronchial lung biopsy, or immunofluorescent staining for PCP.
Histological diagnosis of pulmonary KS is difficult to make. Bronchoscopy seldom obtains large pieces of tissue that are needed to show the characteristic architecture of spindle-shaped cells surrounding thin vascular channels. Diagnostic yield of parenchymal KS by transbronchial lung biopsy was less than 10%. However, the diagnosis of endobronchial KS can be readily made by direct visualisation of endobronchial lesions, which are classically described as violaceous or bright red irregularly shaped lesions that are flat or slightly raised. The indications for bronchoscopy in patients with suspected bronchogenic carcinoma are the same as in HIV-negative subjects.
There is potential risk of transmission of PTB, an airborne infection, to medical personnel during bronchoscopy. Bronchoscopy should only be done in patients with negative sputum AFB smear. Precautions during procedure include use of particulate respirator masks and adequate ventilation in the bronchoscopy suite.
Thoracentesis with or without pleural biopsy should be the done for unexplained pleural effusions. Transthoracic needle aspiration is best reserved for patients with focal peripheral lesions. Lung biopsy obtained by thoracotomy or thoracoscopy is rarely performed but may be needed in difficult cases.
Clinicians may need to decide when to initiate HAART in HIV-infected patients who have developed opportunistic infections such as pulmonary diseases. Initiation of HAART in acute setting could be beneficial by improving immunological and inflammatory responses to opportunistic infections. However, it may cause potential harms. Firstly, patient may develop intense inflammatory responses leading to lung injury. Antiretroviral agents and many common antimicrobial agents have overlapping toxicities, such as skin rash, deranged liver function and cytopaenias. Potential drug interaction is another important issue. Finally, GI absorption of oral antiretroviral agents may be suboptimal in seriously ill patients. Therefore, there is still no consensus when to start HAART in newly diagnosed HIV patients with acute pulmonary diseases.
After HAART is started, patients may experience new clinical symptoms as a result of enhanced immune response, i.e. immune restoration disease (IRD). The true incidence of IRD is not yet clear. It has been described in various pulmonary diseases including PTB, MAC, PCP, KS and lymphoma. The range and severity of clinical manifestations are wide. Some patients have asymptomatic pulmonary nodule or mediastinal lymph node, whereas other patients may develop organ dysfunction such as respiratory failure.
Wallace JM, Hansen NI, Lavange L, et al. Respiratory disease trends in the Pulmonary Complications of HIV Infection Study cohort. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med 1997;155:72-80.
Grubb JR, Moorman AC, Baker RK, Masur H. The changing spectrum of pulmonary disease in patients with HIV infection on antiretroviral therapy. AIDS 2006;20:1095-107.
Hanson DL, Chu SY, Farizo KM, Ward JW. Distribution of CD4+ T lymphocytes at diagnosis of acquired immunodeficiency syndrome-defining and other human immunodeficiency virus-related illnesses. The Adult and Adolescent Spectrum of HIV Disease Project Group. Arch Intern Med 1995;155:1537-42.
Bower M, Powles T, Nelson M, et al. HIV-related lung cancer in the era of highly active antiretroviral therapy. AIDS 2003;17:371-5.
Vander Els NJ, Stover DE. Approach to the patient with pulmonary disease. Clin Chest Med 1996;17:767-85.
Baughman RP. The lung in the immunocompromised patient. Infectious complications Part 1. Respiration 1999;66:95-109.
Naidich DP, McGuinness G. Pulmonary manifestations of AIDs. CT and radiographic correlations. Radiol Clin North Am 1991;29:999-1017.