Kenny CW CHAN
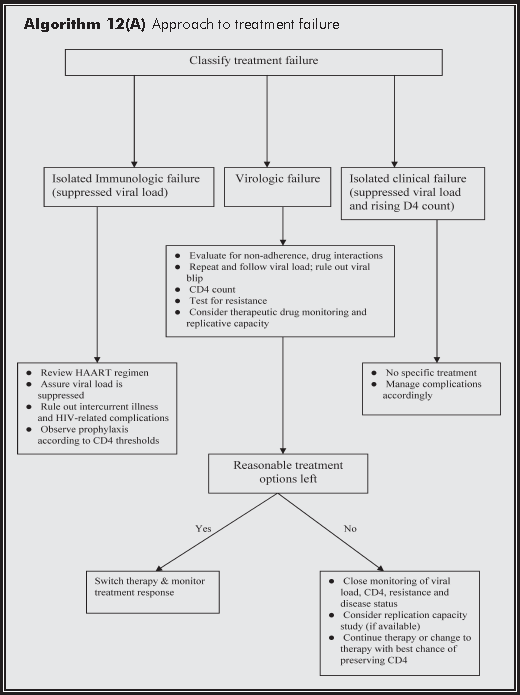
The first highly active antiretroviral therapy (HAART) regimen is designed with a view to long term success. However, this is never guaranteed. For a variety of reasons, treatment fails and has to be modified or even totally changed. Drug resistance is one of the most important causes and also the most difficult to manage. All treatment failure has to be taken very seriously and immediately evaluated by experienced HIV physicians (Algorithm 12(A)).
The basic tenet of antiretroviral treatment is one of complete suppression of viral replication. This theoretically will forestall the development of resistance and damage to the immune system. In this environment, immune recovery follows.
Yet, current treatment now known as HAART may not be that potent. With very sensitive assays, very low level viral replication is still detectable in most patients.1,2 Control of viral replication with HAART as we know it today is therefore a precarious affair, demanding almost perfect adherence as well as superior drug potency. Inadequate HAART will quickly lead to failure. The reasons for inadequate treatment include:
(a) Suboptimal 'HAART' - Antiretrovirals are not identical. Drugs differ in potency, genetic barrier for resistance, prevalence of primary resistance, and toxicity.
(b) Antagonism between some drug combinations.
(c) Inadequate delivery - non-adherence, malabsorption, vomiting, pharmacokinetic interaction.
(d) Excessive metabolism - individual variation, pharmacokinetic interaction.
(e) Pre-existing, archived resistance that failed to be detected in testing.
At the clinical level, it is known that response to HAART is often poorer in patients with advanced disease, who are treatment-experienced, and those with a high baseline viral load and suboptimal drug adherence. In addition, treatment efficacy is usually lower in the real world than in clinical trials where supervision is more intense. Typically, 60-90% of patients achieved successful virologic outcome in clinical trials, whereas in observational cohorts, this proportion was as low as 50-60%.
Treatment failure may be virologic (failure of viral suppression or rebound of plasma HIV-1 RNA), immunologic (falling CD4) or clinical (progression to new AIDS-defining illness). There is no consensus on the precise definition of treatment failure although some criteria have been proposed.
Virologic failure - According to US guidelines,3 virologic failure is present when there is repeated detectable viraemia >400/mL with treatment. In this case, a complete and immediate evaluation is mandatory with possible change to new antiretroviral agents.
Immunologic failure - Immunologic failure is arbitrarily defined as failure of the CD4 count to increase by 25-50/μL or a return to the baseline CD4 before treatment. Most patients will respond immunologically even if viral suppression is incomplete. However, this virologic failure will eventually lead to compensatory mutations and a decline in CD4 count. Until then, the decreased viral fitness secondary to mutations may maintain a temporary discord between virologic failure and immunologic response.
Clinical failure - It is defined as the occurrence or recurrence of HIV-related events after at least 3 months of treatment, with the exception of immune reconstitution syndromes. This definition therefore excludes residual immune deficiency that persists in the early period of antiretroviral therapy. Clinical failure usually follows virologic and immunologic failure in that order.
Virologic failure prompts an evaluation of possible non-adherence and factors that may have contributed to it: adverse effects of drugs, conflict with a patient's lifestyle, pill burden, use of recreational drugs, etc. Concomitant medications such as tuberculosis treatment have to be reviewed for change. Chinese herbs and health foods may be important and should be avoided if there is doubt. The antiretroviral combination in use should also be reviewed for adequacy. It is important that history taking should be thorough and yet non-accusatory, as a compassionate attitude is a pre-requisite to obtain truthful information. Instead of asking 'did you miss any dose?', one may tactfully ask 'have there been times that you could not take your medicines as prescribed?'. The patient should also be evaluated for new onset of HIV-related complications.
Viral load (VL) - Repeated measurements of VL are necessary, as a viral blip should spontaneously resolve. However, any viral load >1000/mL is not a viral blip and should be taken seriously. Occasionally, the pre-treatment VL was so high that an undetectable VL is not evident until 6 months into treatment. In this case, the slope of decrease could be reassuring as the VL should have decreased by at least 1 log at 4 weeks.
CD4 count - The trend and the current level of CD4 count may be important in determining the timing of changing to a new treatment regimen. This is particularly important in patients who do not have viable treatment options. It also indicates the need to start or re-start prophylaxis against opportunistic infections.
Resistance test - Not all failures are due to drug resistance. Yet its occurrence is highly associated with failure to subsequent therapy4,5 and carries a poor prognostic significance.6 Resistance testing by either genotypic or phenotypic methodology has now become the standard of care in treatment failure. It is critical that testing be done when the patient is on the failing treatment or soon after stopping it. Identified mutations will help exclude certain drugs to be used, but absence of mutations does not guarantee success. Since resistance is archived, it is useful that old blood samples be stored and made available for testing in the future when failure occurs. This is especially important for patients who have failed therapy before.
Therapeutic drug monitoring (TDM) - Especially for protease inhibitors, resistance may be relative and can be overcome by a higher level of plasma concentrations. By relating the plasma level to the IC50 of the resistant virus (the inhibitory quotient), simply increasing the dosage may enhance control of the resistant virus. On the other hand, considerable inter-individual variations in the pharmacokinetics of antiretrovirals occur. TDM is however not yet a standard procedure (Chapter 16).
Replicative capacity (RC) - It is currently a research tool, but preliminary data showed correlation with immunologic response and disease progression.7,8 RC is a measurement of viral fitness, a term that describes HIV's adaptability in a given environment, typically in competition with other strains. It is conceivable that in patients with extensive resistance, RC may provide guidance as to whether a failing therapy should be continued (Chapter 16).
A synthesis of information gained from the clinical evaluation and laboratory investigations allows one to reasonably explain the failure and hopefully devise an appropriate change of therapy:
Non-adherence to an otherwise adequate HAART without resistance - In many cases, this would have been preventable if a patient's preference and lifestyle are taken into account in designing the initial regimen. Commonly it is the dosing frequency and pill burden that impair adherence and should be addressed in the new regimen. Adverse effects such as diarrhoea, nausea, or jaundice may also be important and should have been proactively managed with symptomatic treatment and adequate warning. Certain patients may be habitual users of recreational drugs and alcohol, which will make it difficult for them to even remember taking the drugs. Referral to expert help should be considered. Although resistance may not be present in testing, the physician should strive to reasonably conclude that this is the case by ensuring that the resistance testing was timely done and repeating the test on archived or new samples if in doubt. It is important that the patient be adherent to a HAART regimen as soon as possible. This can be achieved by appropriately addressing the reasons behind non-adherence and continuing the same regimen. Alternatively, one may substitute components of therapy that are hindering adherence.
Non-adherence to an otherwise adequate HAART with resistance - Change of therapy in this circumstance will have to address resistance as well as non-adherence. In first treatment failure, adequate treatment options are usually available. However, the patient will have to understand that second line therapy is usually not as tolerable to most patients. If the CD4 count is high, a temporary drug holiday before change to the new therapy is acceptable. This will allow the patient and his care provider to work out issues hindering adherence. Ideally, the antiretrovirals should be changed to three new drugs to which the current therapy has no cross resistance. However, this is rarely possible. In any case, the new regimen should have at least two active agents according to resistance test (i.e. a genotypic or phenotypic sensitivity score of >=2).
Drug interactions with or without resistance - In Hong Kong, consumption of Chinese medicine is common and not often regarded as drugs by patients. History taking should be specific and TDM preferably performed. Pharmacokinetic properties of most Chinese herbs are not known and if in doubt patients should be advised to stop them. Multiple medicines also interact with antiretrovirals. Anti-tuberculosis treatment, methadone, and macrolides are some of the common medications that patients with HIV infection may take concomitantly. Adjusting their dosage based on standard recommendations may not be adequate and TDM should also be considered.
Multiple resistance - This is commonly referred to as the salvage situation where a HAART regimen cannot be confidently constructed that will effectively suppress viral load to undetectable levels. Almost all cases are due to inappropriate sequencing or non-adherence of antiretrovirals for an extended period of time. Management is the domain of experienced HIV physicians to whom all such patients should be referred to.
Drugs specifically developed for resistant viruses have been approved. Experience with two of these drugs, T20 and tipranavir (TPV), confirms the dictum that at least two active agents should be included for successful salvage. The attempt to construct a useful regimen sometimes arrives at a combination of more than 4 drugs commonly referred to as mega-HAART. Such regimens usually include at least 2 PI, 1 to 2 NNRTI, several NRTI and T20. Some would add hydroxyurea or mycophenalate mofetil for NRTI potentiation. Pharmacokinetic interactions, such as boosting by ritonavir (RTV), should be exploited to ensure adequate levels are present specific to the resistant virus. Patient motivation is important. Success relies on the possibility that there is at least partial response to some of the drugs being used. Mega-HAART is difficult in terms of adherence, side effects, and potential drug interactions.
With the expanding antiretroviral armamentarium, the likelihood of success for 'second hit' is now reasonable and definitely much greater than that for salvage therapy. Thus, after the first virologic failure, most physicians will expeditiously change therapy after addressing the underling causes contributing to failure. Procrastination with unnecessary repetitions of tests only leads to development of cross-resistance, decline of CD4 count and ultimately clinical deterioration.
In patients with second or higher order of failure, the chance of successful HAART is significantly diminished. If the new regimen does not contain at least two active agents, resistance is expected to occur as it essentially amounts to monotherapy. In the presence of a high CD4 count, it is acceptable to interrupt therapy and wait for the availability of newer agents that can be included into a successful regimen.
In a true salvage situation where there is no effective HAART, incomplete virologic suppression is no longer realistic. Therefore, treatment goals should be realigned to preservation of CD4 count and prevention of clinical deterioration. To this end, partial viral suppression becomes the immediate objective of therapy. Its rationale is based on impaired viral fitness with resistance mutations.9 This 'cost' to the virus is particularly relevant with PI and NRTI mutations.10 Mutations to NNRTI do not generally impact on fitness and this class of drugs should not be continued. On the other hand, it is known that M184V, the signature mutation to lamivudine (3TC) and emtricitabine (FTC), impairs RT processivity and hence viral fitness. Continuing 3TC and drugs with similar properties is therefore advantageous even in the face of resistance.11 In salvage therapy, viral loads should be frequently monitored, as a rising viral load may indicate progressive compensation of the virus and an impending fall in CD4 count. RC study, if available, may be useful for selection of a regimen that has the biggest impact on viral fitness. There are investigational drugs in clinical trials and attempt should be made to refer these patients for enrolment.12
Strategic treatment interruption to allow overgrowth of wild type virus susceptible to antiretrovirals is not recommended, as virologic suppression, complete or incomplete, is only temporary. Archived resistance invariably emerges. All in all, there will be a net loss of CD4 count compared to continuing the failing therapy.
Most immunologic failure follows virologic failure. The opposite situation where viral load is suppressed but the CD4 count fails to rise sometimes occurs. It is important to rule out laboratory and clerical error in processing the blood samples. Certain non-B subtypes are not accurately measured by the earlier generation of assays and alternative methodologies may need to be considered. Intercurrent illness and opportunistic infections may depress the CD4 count and have to be ruled out. Tenofovir (TDF) + didanosine (ddI) as a backbone of HAART is also known to have a negative impact on immunologic recovery.
In most cases, the phenomenon cannot be explained. Watchful follow-up is probably the only recourse while prophylactic treatment according to usual thresholds will have to observed. Most of these patients do well. Specific immunologic treatment such as IL-2 could be considered, but is overall clinical impact is unknown. Recently, certain antiretroviral combinations were found to be associated with a better immunologic recovery than others.13,14 Its relevance to this situation, however, is unclear.
On the other hand, immune reconstitution disease occasionally occurs in patients who have apparently responded to HAART, and yet deteriorates clinically (see Chapter 15). Immune reconstitution disease is not regarded as 'clinical' failure. It requires specific treatment and sometimes non-steroidal anti-inflammatory drugs and systemic steroids for symptomatic control. HAART is generally continued unless life-threatening disease occurs. True clinical failure develops rarely with virologic success and immunologic response. However, there is evidence that certain HIV-related complications are developing at higher CD4 strata in the HAART era. This is probably related to the fact that immunologic recovery from HIV-mediated damage is not complete despite viral suppression.
Zhang L, Ramratnam B, Tenner-Racz K, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med 1999;340:1605-13.
Dornadula G, Zhang H, VanUitert B, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 1999;282:1627-32.
DHHS. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. May 4, 2006. Available from http://www.AIDSinfo.nih.gov (accessed June 27, 2006).
Lorenzi P, Opravil M, Hirschel B, et al. Impact of drug resistance mutations on virologic response to salvage therapy. Swiss HIV Cohort Study. AIDS 1999;13:F17-21.
Piketty C, Race E, Castiel P, et al. Efficacy of a five-drug combination including ritonavir, saquinavir and efavirenz in patients who failed on a conventional triple-drug regimen: phenotypic resistance to protease inhibitors predicts outcome of therapy. AIDS 1999;13:F71-7.
Welles SL, Jackson JB, Yen-Lieberman B, et al. Prognostic value of plasma human immunodeficiency virus type 1 (HIV-1) RNA levels in patients with advanced HIV-1 disease and with little or no prior zidovudine therapy. AIDS Clinical Trials Group Protocol 116A/116B/117 Team. J Infect Dis 1996;174:696-703.
Daar ES, Kesler KL, Wrin T, et al. HIV-1 pol replication capacity predicts disease progression. AIDS 2005;19:871-7.
Sarmati L, Nicastri E, Montano M, et al. Decrease of replicative capacity of HIV isolates after genotypic guided change of therapy. J Med Virol 2004;72:511-6.
Lucas GM, Gallant JE, Moore RD. Relationship between drug resistance and HIV-1 disease progression or death in patients undergoing resistance testing. AIDS 2004;18:1539-48.
Deeks SG, Hoh R, Neilands TB, et al. Interruption of treatment with individual therapeutic drug classes in adults with multidrug-resistant HIV-1 infection. J Infect Dis 2005;192:1537-44.
Campbell TB, Shulman NS, Johnson SC, et al. Antiviral activity of lamivudine in salvage therapy for multidrug-resistant HIV-1 infection. Clin Infect Dis 2005;41:236-42.
Gandhi T, Wei W, Amin K, Kazanjian P. Effect of maintaining highly active antiretroviral therapy on AIDS events among patients with late-stage HIV infection and inadequate response to therapy. Clin Infect Dis 2006;42:878-84.
DeJesus E, Herrera G, Teofilo E, et al. Abacavir versus zidovudine combined with lamivudine and efavirenz, for the treatment of antiretroviral-naive HIV-infected adults. Clin Infect Dis 2004;39:1038-46.
Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 2006;354:251-60.