Kenny CW CHAN
Tests for HIV differ in methodology as well as testing purpose. Generally, tests are done for three reasons: individual diagnosis, protection of blood or tissue products safety, and public health surveillance. According to the objective of testing, the most appropriate test is chosen based on convenience, test characteristics, and the population to whom the testing subject belongs.
Presence of antibody to HIV proteins is now well accepted as indicative of HIV infection except in extenuating circumstances such as after receiving an experimental vaccine. Certain clinical conditions may also rarely result in false-positive HIV antibody tests. Over the years, experience with the serologic tests and knowledge of viral subtypes helped refine testing algorithms that have high sensitivity and specificity for the purpose of clinical diagnosis. In general, a highly sensitive test is used for screening, followed by a highly specific test for confirmation.
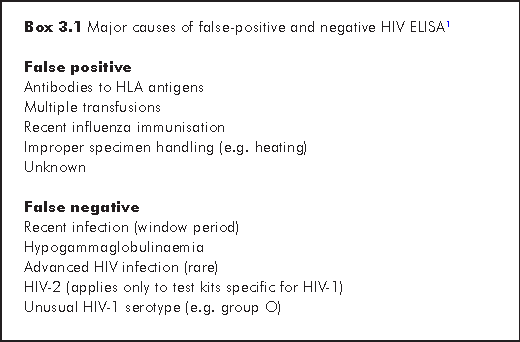
Antibodies to HIV proteins may be detected by various methodologies; the current standards are enzyme-linked immunosorbent assays (ELISAs) for screening and Western blot (WB) for confirmation. Other acceptable methodologies are particle cell agglutination for screening, and immunofluorescence and radioimmunoblot assay for confirmation. ELISA is highly sensitive but has been fraught with the major issues of false positives resulting from insufficient specificity and false negatives mainly from low levels of antibody in very early infection, the so-called window period (Box 3.1). With each successive generation of assays, the rate of false positives has fallen and the window period shrunk significantly. This improvement owes itself to the replacement of viral lysate by recombinant antigens and the use of double-antigen sandwich to enhance capture of IgM and IgG antibodies. Its overall performance characteristics compare very favourably with the best diagnostic tests in other areas of medicine.

In a low prevalence community like Hong Kong, there is an unacceptably high rate of false positive if ELISA is used alone for diagnosis. To improve specificity, the standard algorithm of HIV diagnosis is the tandem use of ELISA followed by WB. The latter has superb specificity and is used for ruling in the diagnosis when a sample tests positive with ELISA. To lower false positives with ELISA, most laboratories re-test the same sample with another ELISA test or a particle agglutination test before it is subjected to confirmation. In some laboratories in Hong Kong, RIBA is also used for confirmation, which has comparable performance to WB. The most commonly used criterion of a positive WB result is the presence of any two bands corresponding to p24, gp41 and gp120/160.2 The presence of only one band to these antigens amounts to an indeterminate result. Single p24 reactivity has rarely been associated with non-Hodgkin's disease and autoimmune diseases such as systemic lupus erythematosus, Sjogren's syndrome, Hashimoto's thyroiditis. Indeterminate results with WB have also been reported with samples with polyclonal gammopathy, haemolysis, rheumatoid factor, and some infections, such as HTLV-1. As indeterminate results may also underlie an early evolving HIV infection, longitudinal follow-up with reassessment of risk of HIV infection and repeat testing is required. Consideration may also be given to performing a virologic test for diagnosis. Confirmation of an HIV-2 positive test is similarly by WB with HIV-2 specific antigens.
It is noted that World Health Organization (WHO) endorses alternative algorithms for use in resource-limited settings, where a positive ELISA result is confirmed by one or two ELISA tests targeting different HIV antigens.3
Currently marketed rapid tests are essentially ELISAs adapted for a rapid turnaround time. Some are intended for point-of-care use. At least four rapid HIV tests have been approved by the US FDA, some of which are available in Hong Kong. Results are available from 5 to 20 minutes. They are interpreted visually and require no instrumentation. The Oraquick® Advance Rapid HIV-1/2 Antibody test (Orasure Technologies) is appropriate for point-of-care use with whole blood or oral fluid, whereas Reveal® G-2 Rapid HIV-1 Antibody Test (MedMira) is categorised as a test of moderate complexity because it requires serum or plasma and hence should be performed by persons with formal laboratory training. Strict adherence to manufacturers' instructions is imperative to assure accurate results. The sensitivity of Oraquick® is 99.6% and specificity 100%, but performance may be somewhat lower in the field, especially with oral fluid.4
Rapid tests may be applied in the following settings:5
(a) Antenatal clinics when the mother presents late. Rapid test supports decision in the use of antiretroviral therapy to prevent mother-to-child transmission of HIV.
(b) Settings where a high risk of needle-stick injury is expected. Rapid test of the source patient and victim, with informed consent, informs the decision to use prophylaxis.
(c) Outreach setting where conventional HIV testing may not reach certain vulnerable populations such as drug users, sex workers and men having sex with men.
(d) Conventional HIV care setting where there is a high defaulter rate. Instead of the traditional two sessions, only one is required for those who test negative.
Nevertheless, it must be noted that:
(a) Rapid test is but one format of ELISA and requires confirmation by WB for diagnosis. Phlebotomy is still required for those with positive rapid test results.
(b) The test is not intended for use by the general public. It is done by medical professionals and requires the same standard as conventional HIV antibody tests. However, counselling techniques may need to be adapted depending on the settings.
The p24 protein is a product of the gag gene and resides in the viral core. Prior to the availability of viral load testing, p24 was used for monitoring disease activity as well as diagnosis. It is specific as a diagnostic test but falls short on sensitivity, as a negative test is not uncommon in genuine HIV infection. In general, the level of p24 rises early soon after infection but subsequently falls as specific antibody develops, only to rise again in the advanced stage of AIDS. Nevertheless, its presence generally precedes that of antibody and will shorten the window period if tested positive. It is now incorporated into the current, fourth-generation ELISA antibody test that is used by the Public Health Laboratory Centre. This generation of ELISA combines testing for both p24 and antibodies to HIV-1 and HIV-2, detects IgM as well as IgG, and has an average window period of only 16 days. The sensitivity is >99.5% and specificity >99% to both B and non-B subtypes. However, there is the theoretical possibility of a brief second window period, during which p24 falls and HIV-antibody remains at low, undetectable levels.6
ELISA and WB are usually performed on serum or plasma. As a result, venipuncture is required, which by itself may be a deterrent to testing. Furthermore, individuals with poor venous access will pose difficulties. Under these circumstances, saliva and urine testing are useful. HIV antibodies, mainly IgG, are present in a lower concentration in these fluids than in serum. Thus, although the same general algorithm can be used, with ELISA followed by WB, it is necessary to employ antibody capture assays to ensure adequate sensitivity. In Hong Kong, universal testing for HIV using urine is carried out in all methadone treatment centres. Urine and saliva tests are also well suited for screening programmes and epidemiologic surveillance by providing a more acceptable alternative to phlebotomy.
Both HIV RNA and proviral DNA are amenable to assay. The major application of RNA assay in HIV medicine is in the measurement of viral load which is essential to gauge the efficacy of antiretroviral therapy. Proviral DNA is incorporated in host cells, of which the peripheral blood mononuclear cells (PBMC) are the most accessible for testing. Both proviral DNA and HIV RNA may be tested for diagnosis. HIV isolation in lymphocyte culture is also diagnostic but has a long turnaround time and places extraordinary demands on facilities and expertise. These virologic tests are mainly used for diagnosis in infants and patients in the window period. The term, Nucleic acid amplification test (NAT), is also used to denote virologic testing algorithms for further reduction of the window period in donated blood.
In infancy and up to the age of 18 months, placentally transferred HIV antibody from the mother precludes antibody testing for diagnosis. Although p24 may be used, its sensitivity in children >=1 month old is suboptimal at 89% even after immune complex dissociation procedures in which antibody to p24 is dissociated from the antigen.7 Virologic tests can definitively diagnose HIV in most infants by the age of 1 month and almost all by 6 months. For example, with the use of HIV DNA PCR, 38% of infected children tested positive by 48 hours of birth, and 93% by 14 days.8 However, this test is not routinely available. Rather, the HIV RNA viral load test is more conveniently employed for clinical diagnosis in infants.9 It is as sensitive as HIV DNA PCR and commonly done by PCR or bDNA in Hong Kong. However, in those with low viral loads, false positives are possible.10 Generally speaking, viral load assay is not recommended for diagnosis. If used, the history should be carefully reviewed, and the possibility of contamination ruled out, as with all diagnostic tests in medicine. If necessary, an HIV specialist or virologist would be a good resource person to consult with. In addition, all positive virologic tests should be repeated for confirmation.
The testing algorithm for infants is described in Chapter 37. Infants with repeatedly negative tests should still be followed by re-testing until HIV infection is excluded by two or more negative virologic tests after the age of 1 month, one of which should be done after 4 months.11
Many of the considerations in paediatric infection are applicable to diagnosis of acute HIV infection. In the window period, antibody testing is usually negative or indeterminate. Diagnosis is thus possible only with virologic tests. Making a diagnosis of acute HIV infection informs proper management and rules out other mimicking syndromes. Infectivity is high in the seroconversion period and counselling on measures to reduce transmission should be given. The potential immunologic benefits of early antiretroviral therapy support the effort to make an early diagnosis, though treatment is advisable only in a clinical trial setting, in the absence of clinical standard.
Nevertheless, indeterminate HIV antibody test results are not uncommon and it is a clinical decision as to whether a virologic test should follow. The presence of features compatible with acute HIV infection, especially after a high risk exposure, will suggest acute infection and repeat testing in a week is advisable. Alternatively, virolgoic tests may be used. However, if symptoms are mild and clinical trials for early treatment of acute HIV infection are not available, one may also comfortably choose to repeat testing for antibody later, after the window period.
Blood borne pathogens in donated blood may escape detection if only serologic markers are used for screening. With the concurrent use of NAT, this window period for HIV detection is reduced to about 11 days. They are implemented as standard in many developed countries, including Hong Kong, to assure the safety of blood supply. In light of the high sensitivity of current serologic assays, the cost-effectiveness of NAT in routine HIV testing is doubtful and will depend highly on the prevalence of HIV in the population.12 It must be noted that NAT complements HIV antibody test, and is rarely used in isolation.
For individual diagnosis, the standard testing algorithm is the two-step use of ELISA for screening and WB for confirmation. The requesting physician should ensure that all tests are done in a quality laboratory.13 An HIV diagnostic test is indicated in people at risk of the infection, including persons with:
(a) Behavioural risks of and potential exposure to HIV infection, such as injecting drug use, any form of cocaine use, male-male sex, multiple sexual partners, and commercial sex. All sex partners of persons with these risk factors are also at risk.
(b) Occupational exposure to a possibly infected source, normally referring to that in the health care setting such as after a needlestick injury.
(c) Other sexually transmitted infections (STI) - It is known that either ulcerative or non-ulcerative STI facilitates HIV transmission.
(d) Clinical conditions associated with HIV infection, varying from classical AIDS-defining conditions to tuberculosis, herpes zoster in a young patient, oral thrush, unexplained fever, and lymphadenopathy.
(e) Receipt of blood or blood products between 1978 and 1985, the year when screening of donated blood and organs began in Hong Kong.
These indications are not meant to be exclusive. With the availability of effective treatment, it is important that an HIV infected person be diagnosed early and referred for medical care to lower morbidity and mortality. In fact, testing should be universally offered in settings that cater mainly for patients at risk of HIV infection, such as the TB and Chest Clinics and Social Hygiene Clinics. HIV testing is also included as part of routine antenatal screening, for the reason that effective therapy is now available to prevent mother-to-child transmission.14,15
Although HIV infection is not a notifiable disease, all medical practitioners are encouraged to report to the Department of Health in an anonymous manner. The reporting form, DH2293, is downloadable from https://www.aids.gov.hk/english/surveillance/form.pdf, and in appendix of this manual.
In clinical practice, an HIV test is performed as a voluntary process. The level of pre- and post-test counselling, information provision (Box 3.2) and the means of obtaining consent may vary with the clinical setting. Test results are to be held in strict confidence. HIV tests in Hong Kong may be performed in private or public service, in hospitals, clinics or even community setting. It's important to note that whoever administers the test for individual diagnosis should also be prepared to give counselling and answer common questions raised by patients, and to make the necessary referral in accordance with the results obtained thereafter. There is no specific requirement as to the qualification of those who deliver counselling in context of HIV test, but the requesting physician shoulders the responsibility to ensure that appropriate and correct counselling be given in a compassionate manner.
The counsellor should take note of the differences inherent with rapid test setting and adapt counselling accordingly. He should assess preparedness of subjects to receive test results in the same setting. Whereas a negative result with rapid test is regarded as definitive, a positive result still requires confirmation by WB. If the existence of the window period is suspected, he should be re-tested in due course. Risk reduction counselling remains important as ever, to avoid unintended reinforcement of risk behaviour.
An individual who tests positive may find it difficult to grasp the meaning of a 'tentatively positive result to be confirmed'. Substantial stress is expected. The counsellor should be prepared not only to give detailed explanation, but to refer the subject to appropriate resources for support.
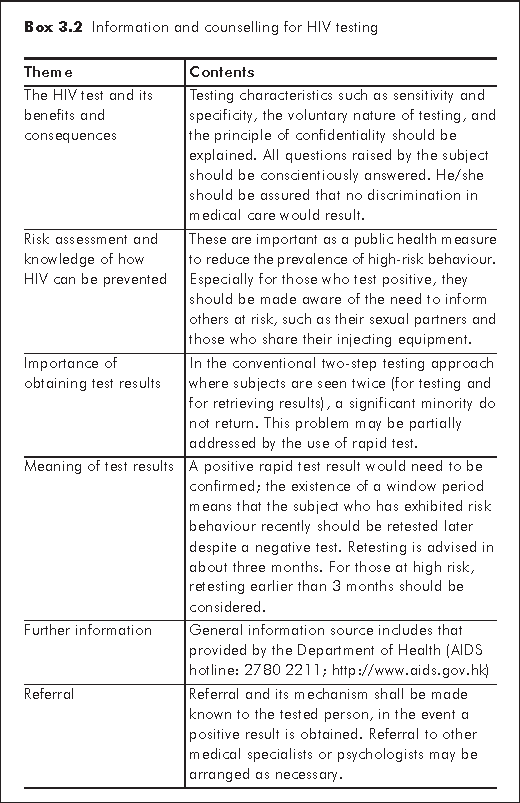
The counsellor should also be prepared to deliver key and concise messages when the test is done in an urgent setting, such as while in labour. In this situation, the need for prophylactic treatment will also have to be explained.
Arens M. Human immunodeficiency virus (HIV) and other human retroviruses. In Essentials of Diagnostic Virology. Churchill Livingstone, 2000:249-70
Centers for Disease Control (CDC). Interpretation and use of the western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. MMWR Morb Mortal Wkly Rep 1989;38(Suppl 7):1-7.
Joint United Nations Programme on HIV/AIDS (UNAIDS)-WHO. Wkly Epidemiol Rec 1997;72:81-7.
US CDC. Supplemental testing for confirmation of reactive oral fluid rapid HIV antibody tests. MMWR 2005;54(Dispatch):1.
Scientific Committee on AIDS. Recommended principles on the application of the HIV antibody rapid test in Hong Kong, December 2003. Available from http://www.aids.gov.hk (accessed June 8, 2006).
Meier T, Knoll E, Henkes M, Enders G, Braun R. Evidence for a diagnostic window in fourth generation assays for HIV. J Clin Virol 2001;23(1-2):113-6.
Quinn TC, Kline R, Moss MW, Livingston RA, Hutton N. Acid dissociation of immune complexes improves diagnostic utility of p24 antigen detection in perinatally acquired human immunodeficiency virus infection. J Infect Dis 1993;167:1193-6.
Dunn DT, Brandt CD, Krivine A, et al. The sensitivity of HIV-1 DNA polymerase chain reaction in the neonatal period and the relative contributions of intra-uterine and intra-partum transmission. AIDS 1995;9:F7-11.
Scientific Committee on AIDS. Recommendations on the management of HIV infection in infants and children, July 2001. Available from http://www.aids.gov.hk (accessed June 8, 2006).
Rich JD, Merriman NA, Mylonakis E, et al. Misdiagnosis of HIV infection by HIV-1 plasma viral load testing: a case series. Ann Intern Med 1999;130:37-9.
Working Group on Antiretroviral Therapy and Medical Management of HIV-infected children. Guidelines for the use of antiretroviral agents in pediatric HIV infection, November 3, 2005. Available from http://aidsinfo.nih.gov (accessed May 23, 2006.
Busch MP, Hecht FM. Nucleic acid amplification testing for diagnosis of acute HIV infection: has the time come? AIDS 2005;19:1317-9.
Scientific Committee on AIDS. HIV antibody testing: recommended measures to generate quality results, 1994. Available from http://www.aids.gov.hk (accessed May 23, 2006).
Scientific Committee of the Hong Kong Advisory Council on AIDS. Recommended clinical guidelines on the prevention of perinatal HIV transmission, 2001. Available from http://www.aids.gov.hk (accessed May 23, 2006).
Ho PL, Chan KC, Chiu SS, et al. Universal antenatal human immunodeficiency virus testing in Hong Kong: consensus statement. Hong Kong Med J 2001;7:421-7.